Abstract
Background
Autism spectrum disorders (ASD) are a heterogeneous group of neurodevelopmental disorders, ranging in severity and characterised by early onset of delay and deviance in the development of social interaction, and verbal and nonverbal communication. ASD is associated with restricted and/or stereotyped interests or behaviours. Tricyclic antidepressants (TCAs) block noradrenaline and serotonin reuptake, increasing the availability of these neurotransmitters in the central nervous system. Via their impact on serotonin, TCAs have been used in the treatment of autistic symptoms and comorbidities in individuals with ASD.
Objectives
To determine if treatment with tricyclic antidepressants:
1) improves the core features of autism, including restricted social interaction, restricted communication, and stereotypical and repetitive behaviours; 2) improves non‐core features such as challenging behaviours; 3) improves comorbid states, such as depression and anxiety; 4) causes adverse effects.
Search methods
We ran the latest searches for this review on 23 May 2011. We searched: Cochrane Central Register of Controlled Trials (CENTRAL), 2011 Issue 2, MEDLINE (1948 to May Week 2, 2011), EMBASE (1980 to 2011 Week 2), PsycINFO (1887 to current), CINAHL (1937 to current). We also searched Dissertation Abstracts International via Dissertation Express, and the metaRegister of Controlled Trials.
Selection criteria
Randomised controlled trials of any dose, duration and frequency of oral TCAs compared with placebo, in children and adolescents with a diagnosis of ASD, where at least one standardised outcome measure had been used.
Data collection and analysis
Two review authors independently selected and appraised the studies for inclusion and risk of bias. All data were continuous.
Main results
Three studies met the inclusion criteria for this review. Two studies used clomipramine and one used tianeptine. All three trials were small, with between 12 and 32 participants. One of the clomipramine trials involved children and young adults, while the other two trials enrolled only children. Due to heterogeneity in study participant characteristics, the TCA medications investigated and the outcome measures used, we were not able to perform any meta‐analysis.
In only one of the three studies was there any indication that giving children tianeptine could be effective in the short term. In this study, parents and teachers reported that it reduced irritability, hyperactivity, inadequate eye contact and inappropriate speech, but clinician ratings found no significant impact on these symptoms. There were also significant adverse effects, including increased drowsiness and reduced activity levels in these individuals while being treated with tianeptine. The evidence of the impact of clomipramine in the two studies is contradictory. There was evidence of improvement in autistic symptoms, irritability and obsessive‐compulsive disorder type symptoms, but conflicting evidence in relation to hyperactivity across the two studies, and no significant changes found with inappropriate speech. There were also adverse effects reported with the use of clomipramine. Although side effect ratings were not significantly different to placebo, there were significant dropout rates in the clomipramine arm of one study.
Authors' conclusions
Clinicians considering the use of TCAs need to be aware of the limited and conflicting evidence of effect and the side effect profile when discussing this treatment option with people who have ASD and their carers. Further research is required before TCAs can be recommended for treatment of individuals with ASD.
Keywords: Adolescent; Child; Humans; Young Adult; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/adverse effects; Antidepressive Agents, Tricyclic/therapeutic use; Child Development Disorders, Pervasive; Child Development Disorders, Pervasive/drug therapy; Clomipramine; Clomipramine/adverse effects; Clomipramine/therapeutic use; Thiazepines; Thiazepines/adverse effects; Thiazepines/therapeutic use
Plain language summary
Tricyclic antidepressant medication for treating children and adolescents with an autism spectrum disorder
Autism spectrum disorders (ASD) are characterised by problems with social interaction and verbal and non‐verbal communication, as well as restricted and repetitive interests and behaviours. Tricyclic antidepressants (TCAs) are medications that alter the level of the neurotransmitter serotonin and have been used in the treatment of autistic symptoms, anxiety and obsessive‐compulsive type behaviours. We found three trials that studied two different TCAs ‐ clomipramine and tianeptine. One of the clomipramine studies involved children and young adults; the other two studies enrolled only children. All three trials were small, with between 12 and 32 participants. There is only limited evidence to support the use of clomipramine or tianeptine in the treatment of individuals with ASD, and some evidence of side effects that would limit their usefulness. Clinicians considering the use of TCAs in ASD need to be aware of the limited and conflicting evidence of effect and the side effect profile of TCAs when discussing this treatment option with patients with ASD and their carers. More research is required before TCAs can be recommended for use in ASD.
Background
Description of the condition
Autism spectrum disorders refer to a continuum of heterogeneous disorders that display varied severity of deficits, ranging from mild to very severe, of which autism is the prototype (Caronna 2008). Autism is a complex neurodevelopmental disorder characterised by the early onset of patterns of delay and deviance in the development of social interaction, verbal and non‐verbal communication and other skills, and is associated with restricted and/or stereotypical patterns of interest or behaviour. The autism spectrum disorders (ASD), which include Childhood Autism or Autistic Disorder, Pervasive Developmental Disorder ‐ Not Otherwise Specified (PDD‐NOS), Atypical Autism and Asperger Syndrome, are usually diagnosed using either the DSM‐IV (APA 2000) or ICD‐10 (WHO 1993) classification systems. Intellectual disability is the most commonly associated condition in people with ASD, with approximately 50% having a comorbid intellectual disability (Nishiyama 2009). However, ASD can include individuals with intellectual functioning ranging from the disability to superior range, and autistic symptoms can be equally severe in those with average and above‐average IQ (Joseph 2002).
Difficulties resulting from ASD are usually lifelong and present a significant burden on the families of autistic individuals and on society, with estimates indicating that only 3% to 10% are able to live independently (Howlin 2004; Billstedt 2007).
Estimates of the prevalence of autism vary between 1 and 40 per 10,000 and for any ASD between 3 and 82 out of 10,000 (from published literature up to April 2004; Williams 2006). Publications from 2006 have estimated the prevalence of any ASD at between 22 and 116 out of 10,000 (Chakrabarti 2005; Fombonne 2005; Baird 2006; Guillem 2006). ASD are three to four times more common in boys than in girls (WHO 1993; Fombonne 1999). It is unclear whether recent suggestions of increasing rates are due to a real increase in the occurrence of the disorder or merely reflect changing study methods, a broadening of the criteria used for diagnosis and increased awareness of ASD.
Description of the intervention
Treatment of ASD is aimed at improving the overall functioning of the child. Behavioural and educational interventions are the main focus of treatment, with pharmacological treatments used as adjuncts (Posey 2001). Pharmacological treatment in ASD may focus on a variety of issues, including core symptoms, associated challenging behaviours and comorbid conditions. For example, people with high levels of repetitive, obsessive‐compulsive type behaviours and mood disorders may experience some relief of their symptoms through the use of pharmacological interventions (Posey 2001).
Tricyclic antidepressants consist of tertiary (amitriptyline, imipramine) and secondary amines (desipramine, nortriptyline) (Sadock 2005). Tricyclic antidepressants block noradrenaline and serotonin reuptake at presynaptic neurons, resulting in increased availability of these neurotransmitters in the central nervous system. After long‐term administration they also impact upon receptor sensitivity, and the number and function of adrenergic and serotonergic receptors, including up‐regulating postsynaptic serotonin receptors (Potter 1998; Sadock 2005). The tertiary amines are more selective for serotonin transport and the secondary amines are relatively more selective for noradrenaline transport.
How the intervention might work
In people with ASD there have been reports of high levels of whole blood and platelet serotonin, an inhibitory neurotransmitter in the central nervous system that is involved in the regulation of multiple functions including mood and emotions (Cook 1996; Hranilovic 2007). Pharmacological interventions are not curative in ASD, but may provide assistance with problematic symptoms or comorbidities. Pharmacological interventions that target the serotonin system may provide assistance with problematic symptoms such as repetitive behaviours similar to those seen in obsessive‐compulsive disorders, stereotyped mannerisms and difficulty with change, or with comorbidities such as affective lability, depression and anxiety (Volkmar 2004).
It has been suggested that TCAs may be useful in the management of patients with ASD through their impact on serotonin. Serotonin is involved in multiple neurobiological pathways that may be altered in people with ASD, including those that mediate mood, social interaction, sleep, obsessive‐compulsive behaviours and aggression. TCAs are usually used for the relief of symptoms of depression and are effective in the treatment of panic and obsessive‐compulsive disorders (particularly the serotoninergic TCAs). Through their effects on serotonin in the central nervous system, it is plausible that tricyclic antidepressants may have an impact on autistic symptoms and the comorbid disorders that these patients experience, improving their quality of life and that of their carers and families. However, the use of tricyclic antidepressants in children and adolescents has been limited by concerns about a narrow therapeutic index and a high toxicity profile. TCAs exert effects on cholinergic, histaminergic and adrenergic receptors, resulting in physical and cognitive adverse effects. Side effects include dry mouth, constipation, urinary retention, blurred vision, sinus tachycardia, impaired cognition, sedation, impaired motor function, weight gain, hypotension, balance problems, impaired co‐ordination and orthostatic hypotension (Peretti 2000). The sedating effects of TCAs can be useful in treating anxiety and insomnia, but some patients find these medications oversedating. Even at therapeutic doses, tricyclic antidepressants have a potent membrane stabilising property, which can lead to cardiac conduction abnormalities, including prolonged PR, QRS and QT intervals, raising the concern of potential cardiotoxicity and sudden death, as well as lowering seizure thresholds (Findling 1999; Peretti 2000). In overdose, tricyclic antidepressants cause cardiac dysrhythmias, central nervous system depression and seizures (Findling 1999). The side effects of tricyclic antidepressants tend not to decrease in severity with long‐term therapy, and may result in reduced compliance and early termination of therapy (Peretti 2000). In addition, due to the effect on central nervous system amine neurotransmitters, tricyclic antidepressant usage may result in mania or aggravation of psychotic symptoms.
Why it is important to do this review
A systematic review of tricyclic antidepressants for the treatment of ASD is required to evaluate their potential benefits and safety.
Objectives
To evaluate the use of tricyclic antidepressants in people with autism spectrum disorders. The objectives are to determine if treatment with tricyclic antidepressants:
improves the core features of autism, including restricted social interaction, restricted communication and stereotypical and repetitive behaviours;
improves non‐core features such as challenging behaviours;
improves comorbid states, such as depression and anxiety; and
causes adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Inclusion was limited to children and adolescents (birth to 18 years of age) with a diagnosis of an autism spectrum disorder (ASD), using a standardised diagnostic instrument (for example, ADOS, ADI‐R, DISCO, CARS) or using established diagnostic criteria as defined by DSM‐IV or ICD‐10, that is Pervasive Developmental Disorder, excluding Rett Syndrome and Childhood Disintegrative Disorder.
Types of interventions
Any oral tricyclic antidepressants, regardless of dosage used, duration of use or frequency of administration, compared with placebo. Tricyclic antidepressants include amitriptyline (amitriptyline hydrochloride), amoxapine, clomipramine (clomipramine hydrochloride), dothiepin (dosulepin hydrochloride or dothiepin hydrochloride), doxepin, imipramine (imipramine hydrochloride), iofepramine, nortriptyline, trimipramine, desipramine, florpiramine, dibenzepin, iprindole, protriptyline and modified tricyclic antidepressants such as tianeptine.
Types of outcome measures
Primary outcomes
Core symptoms of autism, for example, impairments in communication, reciprocal social interaction and behavioural problems, such as repetitive behaviours and rituals, obsessional behaviour and stereotypy.
Non‐core symptoms, including challenging behaviours, sleep disturbance and aggression.
Comorbidities, including depression and anxiety.
Adverse effects.
Secondary outcomes
Parental, child or family quality of life.
Parental or family stress.
We planned to examine short‐term (up to three months), medium‐term (three to 12 months) and long‐term (greater than 12 months) outcomes if the data were available.
We used the primary and secondary outcomes to populate the 'Summary of findings' tables.
Types of measures:
Standardised diagnostic assessment instruments (Childhood Autism Rating Scale, Autism Diagnostic Interview‐Revised, Autism Diagnostic Observation Schedule, Diagnostic Interview for Social and Communication Disorders).
Standardised communication assessments.
Quality of life questionnaires.
Rating scales of emotions and behaviour, including depression, anxiety, aggression, obsessive‐compulsive behaviour and social reciprocity.
Global Clinical Impression Rating Scales.
Other Health Outcome Rating Scale.
Search methods for identification of studies
Electronic searches
We identified relevant trials through electronic searches of the following databases. Searches were first run in July 2009 with no date or language limits. They were updated on 23 May 2011, using date limits to restrict the search to the period since the previous searches.
Cochrane Central Register of Controlled Trials (CENTRAL), part of the Cochrane Library, 2011 Issue 2.
MEDLINE (1948 to May Week 2, 2011)
EMBASE (1980 to 2011 Week 2)
PsycINFO (1887 to current)
CINAHL (1937 to current)
Dissertation Abstracts International
metaRegister of Controlled Trials
The search strategies are in Appendix 1.
Searching other resources
We also searched bibliographies of articles identified through the search strategy and contacted known experts in the field.
Data collection and analysis
Selection of studies
Two authors (RH, RB) independently screened titles and abstracts from the search in 2009 and the search in 2011. Those that did not fulfil the inclusion criteria were discarded. Potentially relevant articles were retrieved for full‐text assessment, assessment of eligibility and data extraction, which the same authors again conducted independently. Disagreements were arbitrated by a third author (SW). The review authors were not blinded to the name(s) of the study author(s), their institution(s) or publication sources at any stage of the review.
Data extraction and management
Data extraction forms were developed a priori and these included information regarding methods, participant details, dose and frequency of tricyclic antidepressant administration, and outcomes. Two authors (RH, RB) extracted data independently using this form. Disagreements were resolved by negotiation with SW. These data were organised using Review Manager 5 (RevMan 2008). We contacted authors for clarification or additional information as necessary. One non‐English language article was identified, and translation was arranged.
Assessment of risk of bias in included studies
In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), two authors (RH, RB) independently evaluated studies for methodological quality and risk of bias against the following criteria: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. Regarding selective reporting, we had a high index of suspicion of selective reporting if the primary outcomes to be assessed in this review were not presented in included trials. We assigned each selected study a judgement relating to the risk of bias for that study by answering a prespecified question about the study's adequacy in relation to each of the key criteria. Judgements were low risk of bias, high risk of bias and unclear risk of bias, which was used if the risk of bias was unknown. There were no disagreements about risk of bias assessments. We also explored any other potential sources of bias, such as stopping a study early or baseline imbalance.
Measures of treatment effect
If two or more studies had been identified that were suitable for inclusion, measured the same outcomes and were considered to be sufficiently homogeneous, we would have performed a meta‐analysis of the results. Meta‐analyses were not completed in this review. If possible in updates we will use odds ratio (OR) in preference to risk ratio (RR) and Mantel‐Haenszel Chi2 statistic (MH) to best handle small sample size.
Dichotomous data
Where outcomes from either standardised instruments or diagnostic evaluations were expressed as proportions, we would have calculated the risk ratio (RR) with 95% confidence interval (CI) from meta‐analysis. We would have calculated number needed to treat if a positive effect for tricyclic antidepressant therapy were found, using the risk ratio estimate and the control risk from the placebo group.
Continuous data
Where standardised assessment tools generated a score as the outcome measure, we made comparisons between the means of these scores. It was felt that it would be ideal to present data from ANCOVA analysis, to take account of potential baseline differences, but that is not always possible. Where only endpoint scores or change scores were available these were used. Where possible, we would have calculated the mean difference as the summary statistic by meta‐analyses. When standard deviations (SDs) or standard errors (SEs) were not available, we extracted P value, t value and CI data so that SDs and SEs could be imputed. Where the same clinical constructs are measured using different scales, we planned to use the standardised mean difference (SMD). The use of a final value or change score did not arise in this review as there was not a situation where both were available. In future reviews if change score or ANOVA analyses that take baseline into account are available, these will be used in preference to final value scores.
Unit of analysis issues
We assessed all included trials to determine the unit of randomisation and whether or not this unit of randomisation was consistent with the unit of analysis. Where cross‐over trials were used, we extracted mean and standard error of paired t‐tests. No unit of analysis errors were identified. In all included studies randomisation, reporting and analysis were conducted as per individual participant.
Dealing with missing data
Where possible, missing data and drop‐outs were assessed and reported for each included study. We intended to report the number of participants included in the final analysis as a proportion of all participants in each study. We reported reasons for missing data where they were provided in the published trials. Where data could not be included in a meta‐analysis, as was the case in this review, we included a qualitative summary in the text of the review.
Assessment of heterogeneity
We assessed consistency of results by visually inspecting the forest plots, by performing the Chi2 test for heterogeneity to assess whether observed differences in results were compatible with chance alone (where a significance level less than 0.10 was interpreted as evidence of heterogeneity) and by examining the I2 statistic (Higgins 2008), a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error. We assessed heterogeneity using the I2 statistic using the current recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity.
Assessment of reporting biases
Insufficient studies were found to use funnel plots to investigate any relationship between effect size and study precision (closely related to sample size).
Data synthesis
Insufficient studies measured the same outcome construct, so meta‐analysis was not possible.The intention was that, had two or more studies been suitable for inclusion and had measured the same outcome construct, that we would have performed a meta‐analysis of the results, initially of all tricyclic antidepressants together and if high heterogeneity had been found we would then have conducted relevant subgroup analysis and meta‐analysis as required. We would have performed both fixed‐effect and random‐effects analyses as part of a sensitivity analysis, but this was not relevant to this review as no meta‐analysis was possible.
For the 'Summary of findings' tables we assessed quality of evidence using the GRADE system (Higgins 2008). The assumed risk for the 'Summary of findings' tables came from the placebo group summary estimate.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were not possible due to a lack of data for meta‐analysis. Anticipated clinical differences had included:
age of participants, i.e. adult versus paediatric;
levels of intellectual disability, i.e. none, mild, moderate, severe;
diagnostic classification ‐ Autistic Disorder/Classical Autism versus Asperger's Disorder versus Pervasive Developmental Disorder‐NOS; and
medication, i.e. different types of tricyclic antidepressants used for treatment, dose.
Sensitivity analysis
Sensitivity analysis was planned to assess the impact of study risk of bias on the results of meta‐analyses. However, it was not possible to do this as the data were not adequate for meta‐analysis.
Results
Description of studies
Results of the search
Electronic literature searches were conducted in July 2009 and yielded 114 titles. The search was updated in May 2011, yielding an additional 47 titles. We excluded 140 articles because they were not randomised controlled trials or were not about participants with ASD. Following full paper review of the remaining 21 articles, three randomised controlled trials, all of which were cross‐over trials, met the inclusion criteria for full data extraction. A further 14 review articles identified in the original literature reviews were searched, but no additional references were identified.
Included studies
Characteristics of participants
Two studies used clomipramine. One of these studies was conducted in children aged four to 15 years in Chicago, USA (Gordon 1993). The other study, conducted in Toronto, Ontario, Canada, involved 23 children and adolescents as well as eight adults and five patients on the border of the adolescent age group (18 and 19 years of age), with participants ranging in age from 10 years to 36 years (Remington 2001). The mean age in this study was 16.3 years, and as the majority of participants were within the child and adolescent age bracket, the authors felt that the results were relevant to this review.
One randomised controlled trial was conducted in children aged four to 15 years in Innsbruck, Austria, using tianeptine (Niederhofer 2003).
Niederhofer 2003 used ICD‐10 diagnostic criteria for Childhood Autism, requiring two independent Child and Adolescent Psychologists to agree on the diagnosis. Gordon 1993 used DSM‐IIIR and Autism Diagnostic Interview (ADI) criteria for Autistic Disorder and Remington used DSM‐IV criteria for Autistic Disorder, requiring independent confirmation of this diagnosis by two investigators.
In two studies, participant IQ varied from 30 to 107 (mean 57) (Gordon 1993) and 35 to 84 (mean 65) (Niederhofer 2003), respectively. The authors of the third study did not report participant IQ or give any other indication of the participants' level of functioning (Remington 2001).
One trial required that all participants be free of any medical or neurological illnesses (Niederhofer 2003). Another required that participants be free of medical illness and have no neurological or genetic causes identified for their diagnosis of Autistic Disorder (Gordon 1993).
Two studies required that their participants be medication‐free for one month (Niederhofer 2003) or three months before the trial was started (Gordon 1993). The third required that the participants had not previously been prescribed clomipramine or haloperidol or, if they had, that an adequate therapeutic trial had not been completed (Remington 2001). The investigators in this study did, however, allow participants to continue taking other psychoactive medications during the trial, with 13 of their 35 participants taking additional medications. In all three trials, the participants continued with their behavioural and educational interventions.
One study had an additional drug‐response specific inclusion criteria. The participants proceeded to the active phase of the trial only if they had a less than 20 percent improvement on behavioural rating scales after the two week placebo run‐in (Gordon 1993).
Types of study
The three included studies used a double‐blind, placebo‐controlled cross‐over study design and all were conducted in outpatient settings. One study included a one week washout phase (Remington 2001). The remaining two studies had a one week taper of one medication while the other was gradually increased in the cross‐over stage, but did not have a washout period (Gordon 1993; Niederhofer 2003). Numbers of participants were small, ranging from 12 (Niederhofer 2003) to 36 (Remington 2001).
Two of the studies involved a third drug comparator. Gordon 1993 included desipramine, a predominantly noradrenergic TCA, in the third arm and Remington 2001 included haloperidol in their third arm. Data were only extracted from the TCA versus placebo branches of each of these studies. Although desipramine is a TCA, the data from this branch of Gordon’s study could not be included in the review as the effects of desipramine were compared to clomipramine and not placebo.
Treatment durations were short, with the clomipramine studies being conducted over five weeks (Gordon 1993) and seven weeks (Remington 2001), and the tianeptine study being conducted over six weeks (Niederhofer 2003).
Types of outcomes
Behaviour
Seven different standardised outcome measures were used in the three included trials (Table 1). Only the Aberrant Behaviour Checklist (ABC) was used in more than one study (Remington 2001; Niederhofer 2003). Niederhofer reported only parent and teacher rating results for the ABC, whereas Remington used clinician ratings of the ABC. It was felt that parent/teacher and clinician observations to assess for treatment effect would be sufficiently different such that these ratings should not be compared directly. Two studies used only clinician ratings but these two studies did not have any common outcome measures (Gordon 1993; Remington 2001; see Table 1).
1. Outcome measures used in included studies.
| Outcome measures | Gordon | Remington | Niederhofer |
| TCA being investigated | Clomipramine | Clomipramine | Tianeptine |
| Ratings conducted by: | Parent‐Teacher Ratings | Clinician Ratings | Clinician Ratings |
| Aberrant Behaviour Checklist (ABC) | x | x | |
| Childhood Autism Rating Scales (CARS) | x | ||
| Childhood Global Assessment Scale (CGAS) | x | ||
| Children's Psychiatric Rating Scale (CPRS) | x | ||
| CPRS‐Autism Relevant Subscale | x | ||
| CPRS‐ OCD and Anxiety Relevant Subscale | x | ||
| Clinical Global Impression Scale | x | x | |
| Symptom Checklist | x | ||
| Subjective Treatment Emergent Symptom Scale | x | ||
| Dosage Treatment Emergenct Symptom Scale (DOTES) | x | ||
| Extrapyramidal Symptom Rating | x |
Side effects
In addition, four scales and checklists were used for recognition of adverse side effects in the three studies. Standardised checklists were used in two studies, namely the Dosage Treatment Emergent Symptoms Checklist (DOTES) and the Extrapyramidal Symptom Rating scale (Remington 2001), and the Subjective Treatment Emergent Symptom Scale (Gordon 1993). One study group designed their own symptom checklists (Niederhofer 2003).
Excluded studies
Eighteen articles were excluded following full paper review. Nine were found not to be randomised controlled trials or did not include a placebo comparison arm in the trial (Kurtis 1966; Campbell 1971; Szekely 1980; Jaselskis 1992; McDougle 1992; Brasic 1994; ; Sanchez 1995; Sanchez 1996; Brodkin 1997). One did not involve patients with the specific diagnosis of an ASD (Aman 1986). Six articles were commentaries, scientific theory papers or case studies (Kehrer 1978; Weizman 1987; Magen 1993; Szabo 1994; Brasic 1997; Oesterheld 1997). One was a conference presentation that presented data from Remington 2001 comparing clomipramine and haloperidol to placebo treatments in patients with ASD, and it was therefore not included (Sloman 1998). For one study there was discussion amongst the researchers regarding inclusion. This study was excluded because it did not randomise the participants to a placebo‐controlled group in comparison with a TCA, but instead all participants completed a two week placebo phase and were subsequently randomised to two intervention groups of clomipramine and desipramine (Gordon 1992). Although desipramine is a tricyclic antidepressant, the data from this study could not be used as there was not a true placebo‐control phase of the study.
Risk of bias in included studies
Allocation
Random sequence generation
Sequence generation was found to be adequate in two of the studies (Gordon 1993; Remington 2001). In the third, participants were reported to be “randomly assigned” but no information was given about how the randomisation process was carried out (Niederhofer 2003). See Figure 1; Figure 2.
1.
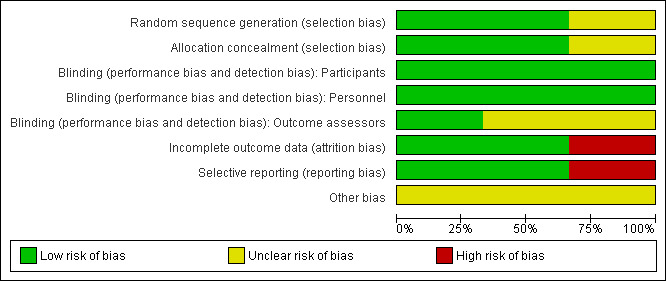
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation concealment
Allocation concealment was adequate in all three studies (Gordon 1993; Remington 2001; Niederhofer 2003).
Blinding
In all three studies, participants and their carers, as well as those involved in medicating the participants, were reported to be blinded to allocation to placebo or intervention phases of the trial (Gordon 1993; Remington 2001; Niederhofer 2003). In one of the studies, it was stated that all involved clinicians who assessed the participants were blind to treatment allocation (Niederhofer 2003). In the other two studies, it was not clear as to whether the assessors were adequately blinded (Gordon 1993; Remington 2001). Both used the term “double‐blind”, but did not specifically report blinding of the assessors. Participants were assessed using the same tools and rating scales during the placebo and treatment phases, but as these were largely observation based measures there is potential for the results to be biased if the assessors were not adequately blind to which phase each patient was in.
Incomplete outcome data
In one of the studies there was no loss to follow‐up (Niederhofer 2003). In one, there was an equal number of participants lost from the placebo and treatment groups (Gordon 1993). In this study each group lost one participant because allocation concealment had to be broken. In the experimental group this was because one patient was given the wrong medication, and in the placebo‐controlled group at the insistence of his parents following violent outbursts. As the numbers were small and the loss of the participants was not related to the study outcomes or due to adverse effects, it was felt that this was unlikely to create bias in the study results. In the third study, incomplete data was not addressed, with the investigators randomising 36 participants to the study but only reporting on 32 of them (Remington 2001). This was a cross‐over study, and there were significant rates of participants dropping out of both the experimental and placebo‐control phases of the study. Twenty of the 32 participants prematurely withdrew from the experimental (clomipramine) phase and 11 of 32 prematurely ended participation in the placebo‐control phase. Reasons for withdrawal from the clomipramine phase of this study included 12 participants who experienced adverse side effects, eight who experienced significant behavioural problems and one participant who had pre‐existing ECG changes. This does potentially add bias to the results reported.
Selective reporting
Two of the three studies were felt to be free of selective outcome reporting (Gordon 1993; Remington 2001). However, there were concerns that there had been reporting of only those outcomes which showed evidence of treatment effect in the third (Niederhofer 2003). Only selected portions of the total number of outcome measures were detailed in the report to show evidence of treatment effect, and these were only those completed by parents and teachers of the participants. The authors did not give details regarding the results of clinician’s assessment data or observation ratings, stating only that “none of the clinician ratings showed significant differences between placebo and treatment”, which is suboptimal. This bias in reporting of results affects the validity of the results of this study.
Other potential sources of bias
Two studies had a one week taper of one medication while the other was gradually increased in the cross‐over stage, but did not have a drug‐free washout period (Gordon 1993; Niederhofer 2003). This did raise concerns that the lack of a washout phase between the placebo and treatment phases of the study may impact upon the results. None of the studies reported any conflict of interest with regard to the funding of the studies.
Risk of bias in cross‐over trials
The main concerns over risk of bias in cross‐over trials are whether the cross‐over design is suitable, whether there is a carry‐over, whether only first period data are available, and incorrect analysis (Higgins 2008). A cross‐over design study can be appropriate in studying ASD, as the condition is reasonably stable, and diagnosis had been made using standardised tools in all three studies. Carry‐over effect of treatment effect across treatment periods may impact upon the risk of bias in all three studies. All three studies used a one week washout or cross‐over period between trial arms to minimise this effect. In addition, Gordon 1993 reported no drug order effect. All three trials analysed data from both the first and second periods of treatment, and where patients did not complete a particular arm of the trial, intention‐to‐treat analysis was carried out. Paired analysis was used, taking advantage of the cross‐over study’s ability to compare treatment effects within individuals. In Remington 2001 and Gordon 1993, repeated measures univariate analysis of variance was conducted comparing clomipramine with placebo and versus baseline scores, and Niederhofer 2003 compared behavioural ratings at baseline, six weeks and 12 weeks using paired, two‐tailed t‐tests. The order of receiving treatments was randomised in all three studies.
Effects of interventions
Due to the level of heterogeneity between study participants, the TCA medications investigated and the outcome measures used by the researchers, meta‐analysis of data could not be performed. Instead a narrative synthesis of the results is presented; see Table 2.
2. Clinically significant outcomes ‐ tricyclic antidepressants compared to placebo.
| Outcome measures | Gordon | Remington | Niederhofer | |
| TCA being investigated | Clomipramine | Clomipramine | Tianeptine | |
| Ratings conducted by: | Clinician Ratings | Clinician Ratings | Parent‐teacher Ratings | Clinician Ratings |
| Aberrant Behaviour Checklist (ABC) | Not significant | Eye contact P = 0.041 Inappropriate speech P = 0.042 Irritability P = 0.047 Hyperactivity P = 0.035 |
Not significant | |
| Childhood Autism Rating Scale (CARS) | Not significant | |||
| Childhood Global Assessment Scale (CGAS) | Not significant | |||
| Children's Psychiatric Rating Scale (CPRS) | P = 0.0001 | Not significant | ||
| CPRS ‐ Autism Relevant Subscale | P = 0.0001 | Overall ‐ not significant Irritability, anger, uncooperativeness P = 0.0001 Hyperactivity P = 0.001 |
||
| CPRS ‐ OCD and Anxiety Relevant Subscale | P = 0.001 | Not significant | ||
| Modified National Institute of Mental Health Global OCD and Anxiety Scale and Modified OCD Scale | Data not provided | |||
| Clinical Global Impression Scale | P = 0.0001 | Not significant | ||
| Symptom Checklist | Not significant | Two participants required dosage modification due to cardiac changes | ||
| Subjective Treatment Emergent Symptom Scale | Not significant | Increased drowsiness P = 0.025 Reduced activity P = 0.029 |
||
| Dosage Treament Emergent Symptom Scales (DOTES) | Not significant, but high drop‐out rates | |||
| Extrapyramidal Symptom Rating | Not significant | |||
Primary outcomes
Global clinical impression
Tianeptine
Clinician ratings based on videotaped interactions between the child and their parent (Niederhofer 2003) were undertaken at baseline, six weeks and 12 weeks using the Children’s Global Assessment Scale, a National Institute of Mental Health Clinical Global Impressions Scale, and a modified Child Psychiatric Rating Scale (CPRS). The authors report that none of the clinician ratings showed significant differences between placebo and tianeptine, after six or after 12 weeks, but no data have been provided.
Clomipramine
Gordon 1993 used the Clinical Global Impressions Scale (CGIA) to rate overall change. The CGIS is a commonly used subjective measure of symptom severity, treatment response and efficacy in pharmacotherapy studies in patients with mental disorders. There was a statistically significant difference in the efficacy index of this scale, which assesses the therapeutic effect of treatments, indicating the superiority of clomipramine to placebo (placebo mean = 11 (SD = 3), clomipramine mean = 5 (SD = 3); F = 27.4; df = 2,22; P = 0.0001).
Core features
Autistic symptoms
Clomipramine
Remington 2001 used the Childhood Autism Rating Scale (CARS) to rate autistic symptomatology. Intention‐to‐treat analysis, using repeated‐measures univariate analyses of variance, was used to compare placebo and clomipramine versus baseline scores. Post hoc comparisons using Scheffe’s F procedure were also used. On intention‐to‐treat analysis of the data comparing placebo to clomipramine a statistically significant difference was found between groups and baseline on the CARS (F(3, 91) = 2.7, P = 0.05), but post hoc comparisons did not indicate a difference from baseline between placebo and clomipramine (baseline mean 41.8 (SD = 7.1), placebo mean = 39.4 (SD = 7.0), clomipramine mean = 37.8 (SD = 8.7)).
Gordon 1993 used repeated‐measures analysis of variance with Bonferroni post hoc tests comparing baseline, placebo and clomipramine treatment. In this study clomipramine was superior to placebo in reducing abnormal behaviours as rated by the Autism Relevant Subscale of the CPRS (placebo mean = 47 (SD = 8), clomipramine mean = 36 (SD = 8); F = 24.2; df 3,33; P = 0.0001). In addition, the items that measure features of autism (including withdrawal, rhythmic motion, abnormal object relationships, unspontaneous relation to examiner, and underproductive speech) indicated a statistically significant difference between treatment with placebo and clomipramine (placebo mean = 23 (SD = 4), clomipramine mean = 18 (SD = 3); F = 22.5, df = 3,33; P = 0.0001).
Abnormal eye contact
Tianeptine
Weekly parent and teacher ratings on the ABC were combined by the trial authors, averaging the scores presented for each subscale, including ‘inadequate eye contact’. Ratings of placebo and tianeptine treatment were compared at baseline, and after six weeks and 12 weeks of treatment, using paired, two‐tailed t‐tests. For this combined parent/teacher score there were improvements in participants’ scores at six weeks (placebo 8.1 (SD = 4.9), tianeptine 7.7 (SD = 3.8), P = 0.052), but these were not statistically significant. At 12 weeks, the improvements from baseline in ratings by parent/teacher reports for inadequate eye contact reached a minimal level of statistical significance (placebo 8.2 (SD = 5.4), tianeptine 7.4 (SD = 3.6), P = 0.041).
The authors report that clinician ratings did not show statistically significant differences between placebo and tianeptine, after six or after 12 weeks on any of the ABC subscales.
Inappropriate speech
Tianeptine
Combined parent and teacher ratings for the ‘inappropriate speech’ subscale of the ABC showed there to be a statistically significant improvement in participants’ scores at six weeks (placebo 6.0 (SD = 2.7), tianeptine 5.2 (SD = 3.5), P = 0.047). At 12 weeks, ratings showing improvements in inappropriate speech remained statistically significant (placebo 6.1 (SD = 2.5), tianeptine 4.2 (SD = 3.8), P = 0.042).
The authors report that clinician ratings did not show significant differences between placebo and tianeptine, after six or after 12 weeks on any of the ABC subscales.
Clomipramine
In one study (Remington 2001) the ABC was scored by clinicians. Repeated measures univariate analysis of variance was conducted comparing clomipramine with placebo and haloperidol. In this report only the results for the arm of the study comparing clomipramine with placebo are considered. The authors first analysed using an intention‐to‐treat approach. There was no statistically significant difference between baseline and treatment with clomipramine for ‘inappropriate speech".
Gordon 1993 used an Autism‐specific subscale of the Child Psychiatric Rating Scale (CPRS) completed by the clinicians, based on parental reports and direct observations. For the speech deviance factors of this measurement there was no statistically significant difference between clomipramine and placebo (placebo mean = 4 (SD = 2), clomipramine mean = 3 (SD = 2); F = 1.4, df = 3,33; P = 0.27).
Stereotypical behaviours
Clomipramine
Remington 2001 used clinician ratings on the ABC, comparing clomipramine with placebo. Intention‐to‐treat analysis showed no statistically significant difference between placebo and treatment groups for stereotypical behaviours.
Non‐core features/behaviour
Obsessive‐compulsive type behaviours
Clomipramine
One study hypothesised that the ritualised, compulsive behaviours seen as features of autism may respond to TCA medications in the same way that the obsessions and repetitive motor behaviours of obsessive‐compulsive disorders would (Gordon 1993). This study used the Modified National Institute of Mental Health (NIMH) Global Obsessive‐Compulsive Disorder (OCD) and Anxiety Scales, the Modified NIMH OCD Scale and the Modified Child Psychiatric Rating Scale (CPSR) OCD Subscale. It found a statistically significant improvement in OCD symptoms with clomipramine as opposed to placebo as measured by the Modified CPRS OCD Subscale (placebo mean = 12 (SD = 4), clomipramine mean = 8 (SD = 4); F = 12.7; df = 3,33; P = 0.001). The authors state that clomipramine was also superior to placebo on the Modified NIMH OCD Scale and the Modified NIMH Global OCD and Anxiety Scales, but did not provide data.
Irritability/anger/uncooperativeness
Tianeptine
Parent and teacher reports on the ABC were combined by averaging the scores. For this combined parent/teacher score there were slight but not statistically significant improvements in participants’ scores for the Irritability subscale at six weeks (placebo 13.8 (SD = 5.1), tianeptine 12.3 (SD = 6.9), P = 0.051). At 12 weeks, the improvements in irritability ratings compared to baseline ratings were statistically significant (placebo 14.2 (SD = 5.4), tianeptine 11.1 (SD = 7.7), P = 0.047). The authors report that clinician ratings did not show significant differences between placebo and tianeptine, after six or 12 weeks on the ABC subscales.
Clomipramine
Remington 2001 analysed ratings on the ABC scored by clinicians. On the intention‐to‐treat data analysis, no statistically significant difference was found between patients’ ratings of irritability at baseline and following treatment with clomipramine.
Gordon 1993 found a statistically significant difference in ratings on the items measuring features of anger and uncooperativeness on the Autism‐relevant subscale of the clinician rated CPRS in the clomipramine treatment phase compared to placebo (placebo mean = 13 (SD = 6), clomipramine mean = 9 (SD = 5); F = 15.9; df = 3,33; P = 0.0001).
Hyperactivity
Tianeptine
Combined parent and teacher ratings for the hyperactivity subscale of the ABC indicated a statistically significant improvement in participants’ scores at 6 weeks (placebo 20.2 (SD = 11.3), tianeptine 19.4 (SD = 9.7), P = 0.048). At 12 weeks, there had been further improvements in hyperactivity ratings (placebo 21.9 (SD = 10.8), tianeptine 19.2 (SD = 11.3), P = 0.035).
The authors report that clinician ratings did not show significant differences between placebo and tianeptine, after 6 or 12 weeks on the ABC subscales.
Clomipramine
In Remington 2001, the ABC was used by clinicians. On both the intention‐to‐treat data analysis and the analysis of data using only results from participants who completed the clomipramine trial, excluding those who dropped out, there was no significant difference between baseline and treatment with clomipramine for ratings of patient hyperactivity.
Gordon 1993 found a statistically significant difference in ratings on the items measuring features of hyperactivity on the Autism‐relevant subscale of the clinician completed CPRS in the clomipramine treatment phase compared to placebo (placebo mean = 8 (SD = 2), clomipramine mean = 6 (SD = 2); F = 8.1; df = 3,33; P = 0.001).
Lethargy
Clomipramine
In Remington 2001, ratings for lethargy on the ABC rating scale performed by clinicians showed no statistically significant difference between baseline and treatment with clomipramine on either intention‐to‐treat data analysis or analysis of the data from participants who completed the clomipramine trial.
Adverse effects
Tianeptine
Parent and teacher weekly ratings on symptom checklists which were averaged found a statistically significant increase in drowsiness at six weeks (placebo 1.4 (SD = 2.3), tianeptine 2.9 (SD = 2.4), P = 0.022) and 12 weeks (placebo 1.5 (SD = 2.8), tianeptine 3.1 (SD = 3.2), P = 0.025).
There was a statistically significant increase in ratings for decreased activity at 6 weeks (placebo 2.6 (SD = 3.7), tianeptine 3.8 (SD = 3.5), P = 0.034) and at 12 weeks (placebo 2.4 (SD = 3.3), tianeptine 4.0 (SD = 3.7), P = 0.029).
Clomipramine
In one study, there was significant drop‐out of participants due to adverse side effects of the medication (Remington 2001). Twelve of 32 participants in the trials were prematurely discontinued with adverse effects identified (fatigue/lethargy N = 4, tremors N = 2, tachycardia N = 1, insomnia N = 1, diaphoresis N = 1, nausea or vomiting N = 1, decreased appetite N = 1). In four of these twelve cases, behavioural problems were also involved in the discontinuation of the trial, and eight more participants were discontinued for this reason specifically. However, on analysis of the data from the Dosage Treatment Emergent Symptom Scale (DOTES) and Extra‐pyramidal Symptom Rating Scale (ESRS), which specifically rated side effects, there were no statistically significant differences between the placebo group and the group treated with clomipramine. The Parkinsonism score for the ESRS was not significantly different between baseline and any of the experimental groups (placebo mean = 7.9 (SD = 7.1), clomipramine mean 10.3 (SD = 7.3), P = 0.35). The behavioural toxicity subscale of the DOTES also found no significant difference between groups (placebo 0.8 (SD = 1.7), clomipramine 2.0 (SD = 2.9), P = 0.07). This study also conducted cardiac monitoring at baseline and at week six of treatment in as many participants as could tolerate this (14 in the clomipramine arm of trial and 15 in the placebo arm), and the analysis of variance indicated no significant changes in ECG variables and no clinically significant arrhythmias were noted.
The second study featuring clomipramine used a Subjective Treatment Emergent Symptoms Subscale to measure adverse side effects (Gordon 1993). There was no statistical significance between clomipramine and placebo in the reporting of adverse effects. The investigators report that two participants required their dosage of clomipramine to be reduced for cardiac considerations – one due to the development of a prolonged QT interval and one developed tachycardia, both of which resolved with this dosage reduction. Reported side effects included insomnia (clomipramine = 7 participants, placebo = 1 participant), constipation (6;2), sedation (6;0), twitching (5;0), tremor (4;1), flushing (4;1), dry mouth (3;1), decreased appetite (3;0) and nausea (2;1).
Comorbidities of ASD
No study used any standardised measure to indicate impact of treatment with TCA on comorbidities of ASD such as depression or anxiety.
Secondary outcomes ‐ parental, child or family quality of life and stress
None of the studies used any standardised measure of quality of life or stress for the child or their carers and families.
Discussion
Summary of main results
TCAs are antidepressants that are used in individuals with ASD. This review details the findings of three randomised, cross‐over controlled trials. Two of the studies evaluated the effect of clomipramine and one study examined tianeptine.
This review includes two studies of children (ages four to 15 years) and one with children and young adults (ages 10 to 36 years), covering a wide range of ages and IQ levels (ranging from 30 to 107). The majority of participants in the studies reviewed had levels of intellectual function in the more severe range of disability.
The review found that there is limited and inconsistent evidence of the effectiveness of the TCAs studied (clomipramine and tianeptine). Using the overall measure of the Clinical Global Impression (CGI) Scale, a commonly used subjective measure of symptom severity, treatment response and efficacy in pharmacotherapy studies in patients with mental disorders, tianeptine did not show a statistically significant difference in outcome to placebo, whereas clomipramine was found to be superior to placebo on CGI ratings (Gordon 1993). The clinical significance of these results is uncertain.
The results of the impact of TCAs on the core features of ASD are inconsistent. Tianeptine was reported to reduce abnormal behaviours such as inadequate eye contact and speech abnormalities more than placebo when rated by parents and teachers, but not when rated by clinicians (Niederhofer 2003). One clomipramine trial reported that clomipramine was superior to placebo in reducing abnormal behaviours as rated by the Autism Relevant Subscale of the CPRS, with specific items that measure features of autism (including withdrawal, rhythmic motion, abnormal object relationships, spontaneous relation to examiner, and underproductive speech) indicating a significant difference between treatment with placebo and clomipramine (Gordon 1993). The other study found no significant differences in ratings of autistic symptoms, speech abnormalities or stereotypical behaviours (Remington 2001). However, in this study only 37.5% of participants completed the clomipramine branch of the trials, limiting the power of the study to find a difference in outcomes.
There were also inconsistent results in relation to TCA and non‐core features of ASD. Clomipramine treatment resulted in statistically significant improvements in obsessive‐compulsive type behaviours (Gordon 1993). One clomipramine trial showed significant improvements in anger and uncooperativeness ratings and ratings of hyperactivity with clomipramine (Gordon 1993), whereas the other showed no difference in either of these symptoms, nor in ratings of lethargy (Remington 2001). Treatment with tianeptine was reported on parent‐teacher ratings to significantly improve irritability and hyperactivity, but clinician ratings did not show any significant difference between tianeptine and placebo (Niederhofer 2003).
There has been limited use of TCAs in the treatment of individuals with ASD, especially in children, due to concerns about possible adverse effects, such as interference with cardiac conduction. Although none of the studies reviewed found statistically significant differences on side effect rating scales between placebo and clomipramine treatment ratings, one study had high dropout rates (20 out of 32 participants) due to adverse effects, with a large number of participants not completing the clomipramine treatment arm of the study (Remington 2001). Of the 20 who discontinued the clomipramine arm of this trial, 12 dropped out due to side effects and eight due to behavioural problems. Remington 2001 was the only study to formally monitor cardiac conduction, doing an ECG at baseline and at six weeks of treatment in as many participants as could tolerate this (baseline N = 23, clomipramine N = 14, placebo N = 15). They found no significant changes in PR, QRS or corrected QT intervals and no clinically significant cardiac arrhythmias. In Gordon 1993, two patients required dose reductions of clomipramine due to the cardiac considerations, one for a tachycardia and one for a prolonged QT interval, but these changes resolved with this dose alteration. Tianeptine resulted in a significant increase in drowsiness and a reduction in activity levels on parent‐completed symptom checklists (Niederhofer 2003).
Overall completeness and applicability of evidence
It is difficult to compare and synthesise information from the studies included in this review for a number of reasons. The diagnosis of ASD was made using a variety of diagnostic criteria and assessment tools, with patients having varying levels of severity of ASD and of intellectual ability. Although the researchers included standardised measures of outcome, they used 11 different outcome measures, only two of which were used across two studies. Gordon 1993 and Remington 2001 used the ABC but involved different assessors in rating outcomes, parents and teachers in one and clinicians in the other. Gordon 1993 and Niederhofer 2003 used the Clinical Global Impression Scale but the medications being investigated were different. Due to an absence of comparable outcome measures of the same TCA in the same participant group, meta‐analysis was not possible.
There were additional limitations of the studies of TCAs in the treatment of ASD identified in this review. All three studies involved small numbers of participants, ranging from 12 to 32, and the majority of participants were male. Small sample sizes increase the risk that no significant change will be found where one exists. ASD is commonly associated with additional features such as sleep disturbance, feeding and dietary difficulties and sensory processing issues, many of which cause significant impairment. The studies reviewed did not measure the impact of TCAs on these associated features. In addition, the duration of each trial was short, and although data were provided at the end of each trial period, the maximum length of follow‐up was 12 weeks, and there was no medium‐ or long‐term follow‐up done in any of the studies. In a chronic condition such as ASD, where treatment durations are likely to be continued for significant periods of time, long‐term data are required to understand the medium‐ and long‐term consequences of medication in this patient population, and these outcomes were not available in any of the studies reviewed.
There are a wide variety of TCAs available for treatment of psychiatric conditions, but only clomipramine and tianeptine were trialled in the included studies.
Quality of the evidence
The overall quality of each study was felt to be adequate, but there were some areas of high risk of bias in each study. Gordon 1993 and Remington 2001 did not provide details about the blinding of outcome assessors. The selection of participants in Gordon 1993, based on a less than 20% response to placebo, could increase the treatment effect size that was identified for the outcomes in which a treatment effect was reported. There were issues with allocation concealment and random sequence generation in Niederhofer 2003, as well as incomplete reporting of outcomes. All three studies were cross‐over study designs, posing a risk of bias, particularly with their short washout periods.
Potential biases in the review process
None known.
Agreements and disagreements with other studies or reviews
There are no other reviews of the use of TCAs in children and adolescents with ASD. It is postulated that TCA medications are effective through their serotonin reuptake inhibition activity, and a Cochrane Systematic Review of selective serotonin reuptake inhibitors (SSRIs) has been conducted (Williams 2010). The authors reviewed seven RCTs, five of which included only children. The conclusion of the review was that there is no evidence of effect of SSRIs in children, and that there was evidence of potential harm through side effects.
Authors' conclusions
Implications for practice.
A limited sample of TCAs have shown small positive effects in children and adolescents with ASD, but the strength of this evidence is negatively impacted upon by the inconsistent findings between studies, the small sample sizes of the studies and their unclear risk of bias, making clear recommendations impossible at this time.
Only two of the many TCAs available for use in individuals with ASD have been evaluated using randomised controlled trials, namely tianeptine and clomipramine. The three studies in this review, two of which assessed the effects of clomipramine, only provide data at the end of short time periods studied, with no evidence of medium or long‐term outcomes and adverse effects.
In one study of tianeptine in children and adolescents, parents and teachers reported perceived reductions in irritability, hyperactivity, inadequate eye contact and inappropriate speech, but clinician ratings found no significant impact on these features (Niederhofer 2003). There were also significant adverse effects, including increased drowsiness and reduced activity levels in these individuals while treated with tianeptine.
In the two studies of clomipramine, the evidence was conflicting. There are adverse effects reported with the use of clomipramine. Although the difference in side effect ratings were not statistically significant compared to placebo, there were high dropout rates in the clomipramine arm of one study (Remington 2001). There was evidence of improvements in ‘autistic symptoms’, irritability and OCD‐like symptoms, but conflicting evidence in relation to hyperactivity, and no significant changes found with inappropriate speech. Clomipramine was not found to reduce 'autistic symptoms' on intention‐to‐treat analyses.
Clinicians considering the use of TCAs in children and adolescents with ASD need to be aware of the limited and conflicting evidence of effect and the side effect profile of TCAs when discussing this treatment option with patients with ASD and their carers.
Implications for research.
This review highlights the limited evidence base regarding the use of TCAs for ASD in children and adolescents.
The evidence base regarding the effectiveness of TCAs for children with ASD needs to be developed by means of adequately powered randomised, placebo‐controlled trials of commonly prescribed and newer TCAs, with sufficient numbers of participants to permit examination of subgroups and their differing responses to these medications, especially differences between paediatric, adolescent and adult patients.
The review highlights the need for a consensus regarding standardised outcome measures that are relevant and specific to ASD. The multiplicity of measures currently in use prevents the meaningful comparison and synthesis of data across trials. Future analytical strategies need to be considered that appropriately take into account baseline differences for optimal data analyses, and should present change scores or ANOVA statistics with baseline taken into account, rather than presenting only final outcome scores.
There are no data on the effect of TCAs on the comorbidities that are commonly associated with ASD, such as anxiety and depression, or on the associated features that may cause significant impairment, such as sleep disturbance, dietary and feeding issues and sensory processing issues. No studies have examined the impact of these medications on the patients’ or carers’ quality of life, so this is highlighted as an area for further investigation.
Although short‐term evidence of benefits and adverse effects are important to our knowledge about the use of TCAs in autism, this is a chronic and heterogeneous condition, and there is a need for trials that examine the long‐term effects of TCA medication in ASD, both positive and adverse. In particular, the risk for children of the membrane stabilising impact on cardiac tissue makes this is an area that requires further study.
Appendices
Appendix 1. Search strategies
CENTRAL #1MeSH descriptor Child Development Disorders, Pervasive explode all trees #2MeSH descriptor Communication, this term only #3autis* or PDD or PDDs or asd or asds or kanner* or asperger* #4pervasive developmental disorder* #5communicat* #6speech near/3 disorder* #7language near/3 delay* #8(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9MeSH descriptor Antidepressive Agents, Tricyclic explode all trees #10TCA #11amitriptyline hydrochloride #12amoxapine #13clomipramine hydrochloride #14(dosulepin hydrochloride or dothiepin hydrochloride) #15doxepin #16 imipramine hydrochloride #17lofepramine #18nortriptyline #19trimipramine #20tricyclic* #21desipramine #22 florpiramine #23dibenzepin #24 iprindole #25protriptyline #26MeSH descriptor Amoxapine, this term only #27Opipramol #28(#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29(#8 AND #28)
MEDLINE (OVID) 1 exp Child Development Disorders, Pervasive/ 2 pervasive developmental disorder$.tw. 3 autis$.tw. 4 kanner$.tw. 5 asperg$.tw. 6 (PDD or PDDs or ASD or ASDs).tw. 7 Communication/ 8 communicat$.tw. 9 (speech adj3 disorder$).tw. 10 (language adj3 delay$).tw. 11 childhood schizophrenia.tw. 12 or/1‐11 13 adolescent/ or child/ 14 (child$ or boy$ or girl$ or teen$ or adolescen$).tw. 15 13 or 14 16 exp Antidepressive Agents, Tricyclic/ 17 (tricyclic$ adj3 antidepres$).tw. 18 TCA.tw. 19 Amitriptyline/ 20 amitriptyline hydrochloride.tw. 21 Amoxapine/ 22 amoxapine.tw. 23 Clomipramine/ 24 clomipramine hydrochloride.tw. 25 Dothiepin/ 26 (dosulepin hydrochloride or dothiepin hydrochloride).tw. 27 Doxepin/ 28 doxepin.tw. 29 Imipramine/ 30 imipramine hydrochloride.tw. 31 Lofepramine/ 32 lofepramine.tw. 33 Nortriptyline/ 34 nortriptyline.tw. 35 Trimipramine/ 36 trimipramine.tw. 37 tricyclic$.tw. 38 Desipramine/ 39 desipramine.tw. 40 florpiramine.tw. 41 dibenzepin.tw. 42 Iprindole/ 43 iprindole.tw. 44 Protriptyline/ 45 protriptyline.tw. 46 or/16‐45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomi#ed.ab. 50 placebo$.ab. 51 drug therapy.fs. 52 randomly.ab. 53 trial.ab. 54 groups.ab. 55 or/47‐54 56 exp animals/ not humans.sh. 57 55 not 56 58 12 and 15 and 46 and 57
EMBASE (OVID) 1 exp autism/ 2 autis$.tw. 3 kanner$.tw. 4 asperg$.tw. 5 childhood schizophrenia.tw. 6 pervasive developmental disorder$.tw. 7 (PDD or PDDs or ASD or ASDs).tw. 8 Communication/ 9 (language adj3 delay$).tw. 10 (speech adj3 disorder$).tw. 11 communicat$.tw. 12 or/1‐11 13 adolescent/ or child/ 14 (child$ or boy$ or girl$ or teen$ or adolescen$).tw. 15 13 or 14 16 exp tricyclic antidepressant agent/ 17 TCA.tw. 18 Amitriptyline/ 19 amitriptyline hydrochloride.tw. 20 Amoxapine/ 21 amoxapine.tw. 22 Clomipramine/ 23 clomipramine hydrochloride.tw. 24 Dothiepin/ 25 (dosulepin hydrochloride or dothiepin hydrochloride).tw. 26 Doxepin/ 27 doxepin.tw. 28 Imipramine/ 29 imipramine hydrochloride.tw. 30 Lofepramine/ 31 lofepramine.tw. 32 Nortriptyline/ 33 nortriptyline.tw. 34 Trimipramine/ 35 trimipramine.tw. 36 tricyclic$.tw. 37 Desipramine/ 38 desipramine.tw. 39 florpiramine.tw. 40 dibenzepin.tw. 41 Iprindole/ 42 iprindole.tw. 43 Protriptyline/ 44 protriptyline.tw. 45 or/16‐44 46 45 and 12 and 15 47 exp Clinical trial/ 48 Randomization/ 49 Single blind procedure/ 50 Double blind procedure/ 51 Crossover procedure/ 52 Placebo/ 53 Randomi#ed.tw. 54 RCT.tw. 55 (random$ adj3 (allocat$ or assign$)).tw. 56 randomly.ab. 57 groups.ab. 58 trial.ab. 59 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 60 Placebo$.tw. 61 Prospective study/ 62 (crossover or cross‐over).tw. 63 prospective.tw. 64 or/47‐63 65 46 and 64
CINAHL (EBSCOhost)
S34 S12 and S16 and S33 S33 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 S32 iprindole S31 protriptyline S30 dibenzepin S29 florpiramine S28 desipramine S27 tricyclic* S26 trimipramine S25 nortriptyline S24 lofepramine S23 imipramine hydrochloride S22 doxepin S21 dosulepin hydrochloride or dothiepin hydrochloride S20 clomipramine hydrochloride S19 amoxapine S18 amitriptyline hydrochloride S17 (MH "Antidepressive Agents, Tricyclic+") S16 S13 or S14 or S15 S15 child* or boy* or girl* or teen* or adolescen* S14 (MH "Child") S13 (MH "Adolescence") S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 communicat* S10 speech N3 disorder* S9 language N3 delay* S8 childhood schizophrenia S7 PDD or PDDs or ASD or ASDs S6 pervasive developmental disorder* S5 kanner* S4 asperg* S3 autis* S2 (MH "Communication") S1 (MH "Child Development Disorders, Pervasive+")
PsycINFO (EBSCOhost)
S28 S9 and S27 S27 S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 S26 protriptyline S25 iprindole S24 dibenzepin S23 floripramine S22 desipramine S21 tricyclic* S20 trimipramine S19 nortriptyline S18 lofepramine S17 imipramine hydrochloride . S16 doxepin S15 dosulepin hydrochloride or dothiepin hydrochloride S14 clomipramine hydrochloride S13 amoxapine S12 amitriptyline hydrochloride S11 TCA S10 DE "Tricyclic Antidepressant Drugs" OR DE "Amitriptyline" OR DE "Chlorimipramine" OR DE "Desipramine" OR DE "Doxepin" OR DE "Imipramine" OR DE "Maprotiline" OR DE "Maprotiline" S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 S8 communicat* S7 speech N3 disorder* S6 language N3 delay* S5 autis* or kanner* or asperg* or childhood schizophrenia* S4 PDD or PDDs or ASD or ASDs S3 pervasive developmental disorder* S2 DE "Communication" S1 DE "Pervasive Developmental Disorders" OR DE "Pervasive Developmental Disorders" OR DE "Aspergers Syndrome" OR DE "Autism" OR DE "Rett Syndrome"
Dissertation Abstracts (via Dissertation Express)
tricyclic*
metaRegister of Controlled Trials (mRCT)
tricyclic* AND autis*
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gordon 1993.
| Methods | Randomised control trial ‐ double‐blind randomised cross‐over comparison | |
| Participants | 7‐15 years of age with DSM‐IIIR and Autism Diagnostic Interview criteria for autistic disorder | |
| Interventions | Clomipramine compared to placebo | |
| Outcomes | Childhood Psychiatric Rating Scale ‐ Autism relevant Subscale Modified Childhood Psychiatric Rating SCale ‐ OCD Subscale Clinical Global Impressions Scale |
|
| Notes | Unusual inclusion criteria. To be included the participants had to have less than 20% improvement on rating scales in the 2‐week single‐blind placebo trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table" |
| Allocation concealment (selection bias) | Low risk | "randomisation was performed by the National Institute of Health Pharmacy" ‐ central allocation by pharmacy |
| Blinding (performance bias and detection bias) Participants | Low risk | "double‐blind randomised crossover comparison". It appears the participants were blinded in regards to the medication and they were assessed in the same way in the treatment and placebo phases. It is likely that the blinding was not broken and outcomes not influenced. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Likely adequate. When blinding was broken for one participant due to violent outbursts and for one who received the wrong medication, they were removed from the trial. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient lost in experimental group due to wrong medication being given. One patient lost in control group due to incorrect medication being given. Equal number of patients lost in each group and loss not related to study outcomes or adverse effects. |
| Selective reporting (reporting bias) | Low risk | The protocol is well described and all expected outcomes are reported. |
| Other bias | Unclear risk | The referral process of self‐ and clinic‐referral may lead to bias as these are potentially self‐selected participants. Risk of bias from cross‐over trial design is low, with appropriate data analysis (repeated measures univariate analysis of variance), and the order of receiving medication was randomised. There was felt to be a small risk of carry‐over effect biasing results as there was a short cross‐over period between treatment arms (one week). |
Niederhofer 2003.
| Methods | Randomised control trial ‐ double‐blind and placebo‐controlled cross‐over study | |
| Participants | Outpatients, aged 4‐15 years, with ICD‐10 criteria for Autistic Disorder, with two independent Child and Adolescent Psychiatrists agreeing on the diagnosis | |
| Interventions | Tianeptine compared with placebo | |
| Outcomes | Parent and Teacher Ratings on Symptom Checklist and Aberrant Behaviour Checklist (ABC) Clinician ratings on Childhood Global Assessment Scale, Modified Childhood Psychiatric Rating Scale (CPRS) and Clinical Gllobal Impression scales |
|
| Notes | Information presented in abstract that is not included in study report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the method of randomisation is given ‐ "the subjects were randomly assigned by a non‐rating clinician to begin tianeptine or placebo". |
| Allocation concealment (selection bias) | Unclear risk | No description given of how patients were allocated, but does say that "tianeptine and identical placebo tablets were administered" |
| Blinding (performance bias and detection bias) Participants | Low risk | Identical medications administered |
| Blinding (performance bias and detection bias) Personnel | Low risk | "All raters (parents, teachers and clinicians) were blind to drug order" |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | "All raters (parents, teachers and clinicians) were blind to drug order" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial so no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Only parents reports were used to report results, and only selected sections of this were reported in the study. There is no mention of what was done with the clinician ratings, except to say "none of the clinician ratings showed significant differences between placebo and tianeptine, after 6 weeks or after 12 weeks". |
| Other bias | Unclear risk | Risk of bias from cross‐over trial design is low, with appropriate data analysis (two‐tailed t‐tests), and the order of receiving medication was randomised. There was felt to be a small risk of carry‐over effect biasing results as there was a short cross‐over period between treatment arms (one week). |
Remington 2001.
| Methods | Randomised control trial | |
| Participants | DSM‐IV diagnosis of Autistic disorder confirmed by two investigators, aged 10‐36 years | |
| Interventions | Three arms to trial ‐ used First Phase data comparing clomipramine with placebo | |
| Outcomes | Childhood Autism Rating Scales (CARS) Aberrant Behaviour Checklist (ABC) Dosage Treatment Emergent Symptom Scales (DOTES) Extrapyramidal Symptom Ratig Scale (ESRS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "subjects were randomly assigned to one of three treatment groups using Latin Square design" |
| Allocation concealment (selection bias) | Low risk | "medications and placebo were packaged in similar capsules to maintain double‐blind component" |
| Blinding (performance bias and detection bias) Participants | Low risk | "medications and placebo were packaged in similar capsules to maintain double‐blind component" |
| Blinding (performance bias and detection bias) Personnel | Low risk | "medications and placebo were packaged in similar capsules to maintain double‐blind component" |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a big loss from all arms of the trial, and although 36 patients were randomised only 32 are reported on. |
| Selective reporting (reporting bias) | Low risk | Reported all that researchers had stated would report. |
| Other bias | Unclear risk | Risk of bias from cross‐over trial design is low, with appropriate data analysis (repeated measures univariate analysis of variance), and the order of receiving medication was randomised. There was felt to be a small risk of carry‐over effect biasing results as there was a short cross‐over period between treatment arms (one week). |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aman 1986 | Did not meet inclusion criteria. Subjects did not have a diagnosis of ASD/Autistic Disorder or PDD‐NOS. |
| Brasic 1994 | Not a randomised control trail and no placebo control. |
| Brasic 1997 | Letter to editor referencing study but is not report of a randomised controlled trial. |
| Brodkin 1997 | Not randomised and no placebo control group. |
| Campbell 1971 | Not a randomised controlled trial. |
| Gordon 1992 | Not randomised to control versus tricyclic antidepressant. |
| Jaselskis 1992 | Not a tricyclic antidepressant. |
| Kehrer 1978 | Not a randomised controlled trial. Discussion paper. |
| Kurtis 1966 | Not a randomised control trial, with no placebo control group used and diagnostic status of participants is unclear. |
| Magen 1993 | Comment in letter to editor, not report of randomised control trial. |
| McDougle 1992 | Case studies, not randomised control trial and no placebo comparison group. |
| Oesterheld 1997 | Case study. |
| Sanchez 1995 | Not randomised, no control group ‐ same patients and pre‐treatment placebo. |
| Sanchez 1996 | Open‐label pilot study, not randomised or placebo‐controlled. Had one week pre‐treatment placebo as baseline for comparison. |
| Sloman 1998 | Contacted author. Data presented from a study which is included among other studies found and excluded. |
| Szabo 1994 | Case report, not RCT. |
| Szekely 1980 | Not a randomised control trial of tricyclic antidepressants versus placebo. |
| Weizman 1987 | Not randomised, no placebo control |
Differences between protocol and review
In the protocol it was stated that no age limitations would be used in the search criteria. Age limitations were introduced to the search criteria, limiting the search to participants in the child and adolescent age ranges. As a result the title of the review has been altered to reflect this.
Contributions of authors
The protocol was prepared by Romy Hurwitz, with input and supervision from Katrina Williams, Roger Blackmore, Sue Woolfenden, Phil Hazell and Danielle Wheeler. Romy Hurwitz and Roger Blackmore independently reviewed titles and abstracts and appraised the quality of papers and extract data. Sue Woolfenden, Katrina Williams and Phil Hazell arbitrated disagreements. All authors contributed to the writing of the review.
Declarations of interest
Romy Hurwitz ‐ none known.
Roger Blackmore ‐ none known.
Philip Hazell ‐ has worked as a consultant for Eli Lilly and Janssen. He has research contracts with Eli Lilly and Celltech. He is a member of the advisory board of Eli Lilly, Australia; Janssen, Australia; Novartis, Australia and Shire, International. Dr Hazell has given presentations for Eli Lilly, Pfizer, Janssen and Sanofi. He is an investigator in a trial of fluoxetine for autism spectrum disorders.
Katrina Williams ‐ I gave a talk about treatments for autism at a symposium organised by Janssen‐Cilag Pty Ltd. Janssen‐Cilag had no control over the contents of the talk and the speaker's fee was paid to the University that employs me. I have no ongoing relationship with Janssen‐Cilag
Susan Woolfenden ‐ none known.
New
References
References to studies included in this review
Gordon 1993 {published data only}
- Gordon CT, State RC, Nelson JE, Hamburger SD, Rapoport JL. A double‐blind comparison of clomipramine, desipramine and placebo in the treatment of Autistic Disorder. Archives of General Psychiatry 1993;50(6):441‐7. [DOI] [PubMed] [Google Scholar]
Niederhofer 2003 {published data only}
- Niederhofer H, Staffen W, Mair A. Tianeptine: A novel strategy of psychopharmacological treatment of children with autistic disorder. Human Psychopharmacology 2003;18(5):389‐93. [DOI] [PubMed] [Google Scholar]
Remington 2001 {published data only}
- Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: a double‐blind, placebo‐controlled, crossover study. Journal of Clinical Psychopharmacology 2001;21(4):440‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aman 1986 {published data only}
- Aman MG, White AJ, Vaithianathan C, Teehan CJ. Preliminary study of imipramine in profoundly retarded residents. Journal of Autism and Developmental Disorders 1986;16(3):263‐73. [DOI] [PubMed] [Google Scholar]
Brasic 1994 {published data only}
- Brasic JR, Barnett JY, Kaplan D, Sheitman BB, Aisemberg P, Lafargue RT, et al. Clomipramine ameliorates adventitious movements and compulsions in prepubertal boys with autistic disorder and severe mental retardation. Neurology 1994;44(7):1309‐12. [DOI] [PubMed] [Google Scholar]
Brasic 1997 {published data only}
- Brasic JR, Barnett JY, Sheitman BB, Tsaltas MO. Adverse effects of clomipramine. Journal of the Americam Academy of Child and Adolescent Psychiatry 1997;36(9):1165‐6. [DOI] [PubMed] [Google Scholar]
Brodkin 1997 {published data only}
- Brodkin ES, McDougle CJ, Naylor ST, Cohen DJ, Price LH. Clomipramine in adults with pervasive developmental disorders: A prospective open‐label investigation. Journal of Child and Adolescent Psychopharmacology 1997;7(2):109‐21. [DOI] [PubMed] [Google Scholar]
Campbell 1971 {published data only}
- Campbell M, Fish B, Shapiro T, Floyd A. Imipramine in preschool autistic and schizophrenic children. Journal of Autism and Childhood Schizophrenia 1971;1(3):267‐82. [DOI] [PubMed] [Google Scholar]
Gordon 1992 {published data only}
- Gordon CT, Rapoport JL, Hamburger SD, State RC, Mannheim GB. Differential response of seven subjects with Autistic Disorder to clomipramine and desipramine. American Journal of Psychiatry 1992;149(3):363‐6. [DOI] [PubMed] [Google Scholar]
Jaselskis 1992 {published data only}
- Jaselskis CA, Cook EH, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with Autistic Disorder. Journal of Clinical Psychopharmacology 1992;12(5):322‐7. [PubMed] [Google Scholar]
Kehrer 1978 {published data only}
- Kehrer HE. Infantile autism and drug therapy [German] [Die medikamentose Behandlung des kindlichen Autismus]. Bibliotheca Psychiatrica 1978;157:91‐7. [PubMed] [Google Scholar]
Kurtis 1966 {published data only}
- Kurtis LB. Clinical study of the response to nortriptyline on autistic children. International Journal of Neuropsychiatry 1966;2(4):298‐301. [PubMed] [Google Scholar]
Magen 1993 {published data only}
- Magen J. Negative results with clomipramine [comment]. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(5):1079‐80. [DOI] [PubMed] [Google Scholar]
McDougle 1992 {published data only}
- McDougle CJ, Price LH, Volkmar FR, Goodman WK, Ward‐O'Brien D, Nielson J, et al. Clomipramine in autism: Preliminary evidence of efficacy. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(4):746‐50. [DOI] [PubMed] [Google Scholar]
Oesterheld 1997 {published data only}
- Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: Shifting metabolic ratios. Journal of Clinical Psychopharmacology 1997;17(1):62‐3. [DOI] [PubMed] [Google Scholar]
Sanchez 1995 {published data only}
- Sanchez LE, Campbell M, Small AM, Adams PB, Uysal S, Hallin A. A comparison of live and videotaped ratings: Clomipramine and haloperidol in autism. Psychopharmacology Bulletin 1995;31(2):371‐8. [PubMed] [Google Scholar]
Sanchez 1996 {published data only}
- Sanchez LE, Campbell M, Small AM, Cueva JE, Armenteros JL, Adams PB. A pilot study of clomipramine in young autistic children. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(4):537‐44. [DOI] [PubMed] [Google Scholar]
Sloman 1998 {published and unpublished data}
- Sloman L. Haloperidol versus clomipramine in Autistic Disorder. 151st Annual Meeting of the American Psychiatric Association. 1998 May‐June; Vol. 17.
Szabo 1994 {published data only}
- Szabo CP, Bracken C. Imipramine and Asperger's. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33(3):431‐2. [DOI] [PubMed] [Google Scholar]
Szekely 1980 {published data only}
- Szekely GA, Caplan R, Rotman A. Platelet dopamine uptake in autistic and other psychotic children. Inhibition by imipramine. Progress in Neuro‐Psychopharmacology 1980;4(2):215‐8. [DOI] [PubMed] [Google Scholar]
Weizman 1987 {published data only}
- Weizman A, Gonen N, Tyano S, Rehavi M. Platelet [3H] imipramine binding in autism and schizophrenia. Psychopharmacology 1987;91(1):101‐3. [DOI] [PubMed] [Google Scholar]
Additional references
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision (DSM‐IV‐TR). 4th Edition. Washington DC: American Psychiatric Association, 2000. [Google Scholar]
Baird 2006
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SCAP). Lancet 2006;368(9531):210‐5. [DOI] [PubMed] [Google Scholar]
Billstedt 2007
- Billstedt E, Gillberg IC, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. Journal of Child Psychology and Psychiatry 2007;48(11):1102‐10. [DOI] [PubMed] [Google Scholar]
Caronna 2008
- Caronna EB, Milunsky JM, Tager‐Flusberg H. Autism spectrum disorders: clinical and research frontiers. Archives of Disease in Childhood 2008;93:518‐23. [DOI] [PubMed] [Google Scholar]
Chakrabarti 2005
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. American Journal of Psychiatry 2005;162:1133‐41. [DOI] [PubMed] [Google Scholar]
Cook 1996
- Cook EH, Leventhal BL. The serotonin system in autism. Current Opinion in Paediatrics 1996;8:348‐54. [DOI] [PubMed] [Google Scholar]
Findling 1999
- Findling RL, Reed MD, Blumer JL. Pharmacological treatment of depression in children and adolescents. Paediatric Drugs 1999;1:161‐82. [DOI] [PubMed] [Google Scholar]
Fombonne 1999
- Fombonne E. The epidemiology of autism: a review. Psychological Medicine 1999;29(4):769‐86. [DOI] [PubMed] [Google Scholar]
Fombonne 2005
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. Journal of Clinical Psychiatry 2005;66:3‐8. [PubMed] [Google Scholar]
Guillem 2006
- Guillem P, Cans C, Guinchat V, Ratel M, Jouk PS. Trends, perinatal characteristics and medical conditions in pervasive developmental disorders. Developmental Medicine and Child Neurology 2006;48(11):896‐900. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Howlin 2004
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcomes for children with autism. Journal of Child Psychology and Psychiatry 2004;45:212‐29. [DOI] [PubMed] [Google Scholar]
Hranilovic 2007
- Hranilovic D, Busjas‐Petkovic Z, Vragovic R, Vuk T, Hock K, Jernej B. Hyperserotonemia in adults with autistic disorder. Journal of Autism and Developmental Disorders 2007;37(10):1934‐40. [DOI] [PubMed] [Google Scholar]
Joseph 2002
- Joseph RM, Tager‐Flusberg H, Lord C. Cognitive profiles and social‐communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry 2002;43(6):807‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishiyama 2009
- Nishiyama T, Taniai H, Miyachi T, Ozaki K, Tomita M, Sumi S. Genetic correlation between autistic traits and IQ in a population‐based sample of twins with autism spectrum disorders (ASDs). Journal of Human Genetics 2009;54:56‐61. [DOI] [PubMed] [Google Scholar]
Peretti 2000
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants versus serotonin reuptake inhibitors. Review. Acta Psychiatrica Scandinavica. Supplementum 2000;101(403):17‐25. [DOI] [PubMed] [Google Scholar]
Posey 2001
- Posey DJ, McDougle CJ. Pharmacotherapeutic management of autism. Expert Opinion of Pharmacotherapy 2001;2(4):587‐685. [DOI] [PubMed] [Google Scholar]
Potter 1998
- Potter WZ, Manji HK, Rudorfer MW. Tricyclics and tetracyclics. The American Psychiatric Press Textbook of Psychopharmacology. 2nd Edition. Washington, DC: American Psychiatric Press, 1998:199‐218. [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Sadock 2005
- Sadock BJ, Sadock VA. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 8th Edition. Lippincott Williams and Wilkins, 2005. [Google Scholar]
Volkmar 2004
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and Pervasive Developmental Disorders. Journal of Child Psychology and Psychiatry 2004;45(1):135‐70. [DOI] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 classification of mental and behaviour disorders: diagnostic criteria for research. Geneva: World Health Organization 1993.
Williams 2006
- Williams JG, Higgins JPT, Brayne CEG. Systematic review of prevalence of autism spectrum disorders. Archives of Disease in Childhood 2006;91:8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2010
- Williams K, Wheeler DM, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD004677.pub2] [DOI] [PubMed] [Google Scholar]


