Abstract
Phenotypic modulation of vascular smooth muscle cells (SMC) is essential for the development of intimal hyperplasia. Lysophosphatidic acid (LPA) is a serum component that can promote phenotypic modulation of cultured SMC, but an endogenous role for this bioactive lipid as a regulator of SMC function in vivo has not been established. Ligation injury of the carotid artery in mice increased levels in the vessel of both autotaxin, the lysophospholipaseD enzyme responsible for generation of extracellular LPA, and two LPA responsive G-protein coupled receptors 1 (LPA1) and 2 (LPA2). LPA1-/-2-/- mice were partially protected from the development of injury-induced neointimal hyperplasia, whereas LPA1-/- mice developed larger neointimal lesions after injury. Growth in serum, LPA-induced ERK activation, and migration to LPA and serum were all attenuated in SMC isolated from LPA1-/-2-/- mice. In contrast, LPA1-/- SMCs exhibited enhanced migration resulting from an upregulation of LPA3. However, despite their involvement in intimal hyperplasia, neither LPA1 nor LPA2 were required for dedifferentiation of SMC following vascular injury or dedifferentiation of isolated SMC in response to LPA or serum in vitro. Similarly, neither LPA1 nor LPA2 were required for LPA to elicit a transient increase in blood pressure following intravenous administration of LPA to mice. These results identify a role for LPA and two defined LPA receptors in regulating SMC migratory responses in the context of vascular injury, but suggest that additional LPA receptor subtypes are required for other LPA-mediated effects in the vasculature.
Phenotypic modulation of vascular smooth muscle cells (SMCs) occurs in response to vascular injury and is a critical component in the development of atherosclerotic and restenotic lesions 1, 2. Changes in the extracellular environment promote this response, which is characterized by alterations in the differentiation state of SMCs and in their acquisition of the capacity to proliferate and migrate. Isolated vascular SMCs from human and rodent species can be stimulated to dedifferentiate, proliferate, and migrate by serum. The lipid mediator, lysophosphatidic acid (LPA), has been proposed as one of the factors present in serum that may promote phenotypic modulation of vascular SMCs 3-5.
LPA, the simplest glycerophospholipid, promotes dedifferentiation3, proliferation 6-8, and migration 9 of isolated vascular SMCs. Although the specific signaling systems involved in SMC responses are not known, in other cell systems, LPA acts through at least five G-protein coupled receptors, termed LPA 1 – 5, to stimulate a wide variety of intracellular signaling pathways important in health and disease 10. Prominent among the pathways that are activated by LPA include Rho GTPases and extracellular-signal regulated kinase (ERK), which are known to play important roles in vascular SMC function. LPA has also been proposed to serve as an endogenous activator of PPARγ 11, and there may be additional, yet-unidentified receptor targets for LPA.
LPA is produced in locations that position it to be a pathophysiologic mediator of vascular cell function. LPA is present in relatively low levels in plasma, but is more abundant in serum where is it thought be derived at least in part through processes that require platelet activation 12-18. Therefore, local concentrations of LPA may be increased along vessels at sites of platelet adhesion and thrombus formation. LPA is also found in abundance in the lipid-rich core of atherosclerotic plaque, where is may be derived from mildly oxidized LDL19. Thus, LPA is present, or can be formed, in the settings associated with alterations in SMC function. The lysophospholipase D autotaxin (ATX), which catalyzes the hydrolysis of lysophospholipid substrates, is responsible for generation of biologically active LPA in circulation. Mice that are heterozygous for the wild type and null ATX allele have 50% normal circulating LPA levels 20, 21 and mice that transgenically overexpress ATX in the liver under control of the α-antitrypsin promoter have elevated circulating levels of ATX and LPA 22.
Exogenous administration of LPA to animals elicits responses consistent with it serving as an endogenous mediator of vascular cell function. For example, intravenous injection of LPA elevates arterial blood pressure in rats 23 and local application causes cerebral vasoconstriction in pigs 24. Moreover, local infusion of LPA in the rat common carotid artery induces vascular remodeling by stimulating neointimal formation 25. A similar response is observed in mice and may be mediated by PPARγ 26.
Until recently, a lack of appropriate and specific tools has limited our ability to define pathophysiologic roles of endogenous LPA in the vasculature. In particular, the most extensive in vitro studies reported to date have used rat or human SMC while responses of murine SMC to LPA have not been examined in detail. A concerted analysis of murine SMC responses to LPA in vitro coupled with a phenotypic analysis of vascular injury responses of LPA receptor deficient mice is required to provide definitive insights into the role of LPA in this important aspect of vascular function. In this report, we identify an upregulation of ATX and LPA receptors 1 and 2 (LPA1 and LPA2) following vascular injury. We use mice deficient in LPA1 and 2 alone and in combination to define roles for these receptors and LPA in the pathophysiologically relevant vascular responses and in the phenotypic modulation of SMCs that occurs with vascular injury.
Methods
Mice
All procedures conformed to the recommendations of “Guide for the Care and Use of Laboratory Animals” (Department of Health, Education, and Welfare publication number NIH 78-23, 1996) and were approved by the Institutional Animal Care and Use Committee. The production and initial characterization of mice-deficient in LPA receptors 1 and 2 has previously been described 27, 28. The mice were backcrossed for >10 generations to the BalbC background. Mice were housed in cages with HEPA-filtered air in rooms on 12-h light cycles and fed Purina 5058 rodent chow ad libitum. Systolic blood pressure and heart rate were measured for five consecutive days in conscious mice using the Blood Pressure Analysis tail cuff system (Hateras Systems, Apex, NC) daily after training for one week. Mean intraartrial pressure was measured by placement of a 1.4 Fr Millar catheter in the carotid artery of isoflurane-anesthetized mice.
Vascular Injury
At various intervals after carotid surgery29, 30, 5 mm of aorta proximal to the suture were removed and processed for RNA analysis by qualitative PCR (qPCR) or analysis of protein markers by immunoblotting. Neointimal formation along the length of the vessel was assessed at four weeks after surgery using computer assisted morphometry as has been previously described 30. Digital images were taken with a high performance digital camera (resolution 3840 × 3072 pixels) attached to a Nikon 80i microscope with a 10× (NA = 0.3) or 20× (NA = 0.5) objective and analyzed with Metamorph software. Femoral artery denudation injury was performed and analyzed at four weeks as previously described 31, 32.
Isolation of SMCs
Mouse aortic SMCs were obtained from thoracic aortas by removing the adventitia and endothelium by digestion with collagenase type II (Worthington; 175 units/ml). The media were further digested in solution containing collagenase type II (175 units/ml) and elastase (Sigma; 0.5 mg/ml), which yielded ≈100,000 cells per aorta. Cells were grown in DMEM containing 0.5 ng/ml EGF, 5 μg/ml insulin, 2 ng/ml bFGF, 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin, and incubated at 37°C with 5% CO2/95% air. SMC lineage was confirmed by the presence of immunoreactivity for α-actin (Sigma) in >99% of the cells. Experiments involving SMCs were performed by using cells with a passage number ≤5.
Details of methods for SMC experiments can be found in Online Supplemental methods.
Statistical analysis
All results were expressed as mean ± standard error of the mean (SEM). In vitro studies were repeated a minimum of three times and results analyzed by student t-test or ANOVA. Statistical significance within strains was determined using ANOVA with multiple pair-wise comparisons. Statistical analysis was performed using Sigma-STAT software, version 3.5 (Systat Software, Inc.). A p-value of less than 0.05 was considered significant.
Results
Exposure of isolated smooth muscle cells 3 or intact vessels 25 to exogenous LPA elicits SMC phenotypic modulation; however the role of endogenous LPA in mediating SMC responses in the context of vascular injury is not known. The aim of this study was to determine if LPA contributes to vascular injury responses in intact organisms. To accomplish this aim, we used mice deficient in candidate LPA receptors and pharmacological antagonists of these receptors to attenuate normal LPA signaling in vascular SMC and determine the consequences on vascular and isolated cells responses.
Upregulation of autotaxin (ATX) and LPA receptors following vascular injury
Following ligation injury, we observed a time-dependent increase in vessel-associated levels of ATX protein that was ∼2.5 fold after 1 - 3 days (p<0.05 at 1 day and 3 days versus uninjured; Figure 1A). ATX is a secreted lysophospholipase D responsible for generation of biologically-active extracellular LPA. Increasing plasma levels of ATX elevates circulating LPA levels 22, therefore, extracellular LPA production along the vessel wall likely increases following injury. In many systems, LPA elicits cellular effects via G-protein-coupled receptor signaling. We therefore assessed the level of gene expression of the five known LPA receptors (LPA 1 – 5) following injury and observed that expression of LPA1 and LPA2 increased following carotid injury by 2.1 fold and 6.2 fold, respectively (Figure 1B).
Figure 1. Increases in autotaxin/lysophospholipase D and LPA receptor expression following arterial injury.
Carotid arteries were isolated from control, uninjured mice or animals that underwent carotid ligation. Autotaxin (ATX) expression was determined by immunoblotting and was significantly higher (p<0.05 by ANOVA) at one and three days after injury n comparison to uninjured control levels (A). LPA receptor expression was determined by quantitative PCR (B). Results are expressed relative to uninjured vessel and presented as mean ± sd from 3 separate experiments.
LPA1-/-2-/- mice are partially protected from development of intimal hyperplasia in vascular injury models while LPA1-/- mice exhibit positive remodeling characterized by an enhanced neointimal response
Mice that are singly or doubly deficient in LPA1 and LPA2 (LPA1-/-, LPA2-/- and LPA1-/-2-/- mice) are viable and fertile 27, 28, allowing us to use them to investigate a role for these LPA signaling receptors in the regulation of vascular SMC responses. Carotid ligation was performed in wild-type (WT) mice, LPA1-/-, LPA2-/- and LPA1-/-2-/- double knock-out (DKO) mice. Four weeks after carotid ligation, neointima developed along the length of the carotid in WT mice (Figure 2A and 2B). The extent of intimal area and the intima/media ratio was similar in mice lacking LPA2 (Figure 2B and 2D). In contrast LPA1-/- mice displayed an ∼ 3-fold enhanced intimal area (Figure 2B), an increase in intima/media ratio (p <0.017 versus WT; Figure 2D), a larger lumen area (Figure 2E), and a resulting increase in the area within the external elastic lamina, consistent with positive remodeling (i.e maintenance of luminal area) in the presence of heightened neointimal formation. Unlike either the LPA2-/- or LPA1-/- mice which displayed normal and enhanced neointima, respectively, LPA1-/-2-/- DKO were partially protected from the development of intimal hyperplasia with an ∼4-fold lower intimal areas (p<0.05; Figure 2B) and intima/media ratios after carotid ligation (Figure 2D). Medial areas were similar in all the mice (Figure 2C).
Figure 2. Alterations in the development of intimal hyperplasia in mice lacking LPA receptors.
Representative CME-stained sections of carotid arteries at four weeks after surgery taken approx 1.2 mm from the site of ligation in WT, LPA1-/-, LPA1-/-2-/- mice (A). Intimal area (B), medial area (C), and intima/media ratio (D) and luminal areas (E) in μm2 along the length of vessels in WT (WT; n = 8), LPA1-/- (n = 6), LPA2-/- (n = 6), LPA1-/-2-/- (n = 6) mice. * = p<0.05 in comparison to WT by ANOVA with multiple pairwise comparisons.
To determine if these results were model specific, we denuded the endothelium of the mouse femoral artery to elicit the development of intimal hyperplasia and observed an enhanced injury response that was broadly similar to that observed in the carotid artery ligation model in LPA1-/- mice (see Online Figure 1). Additional experiments were not performed with the other strains of LPA receptor deficient mice using this femoral artery model because of a high incidence of acute arterial thrombosis in WT Balb/C.
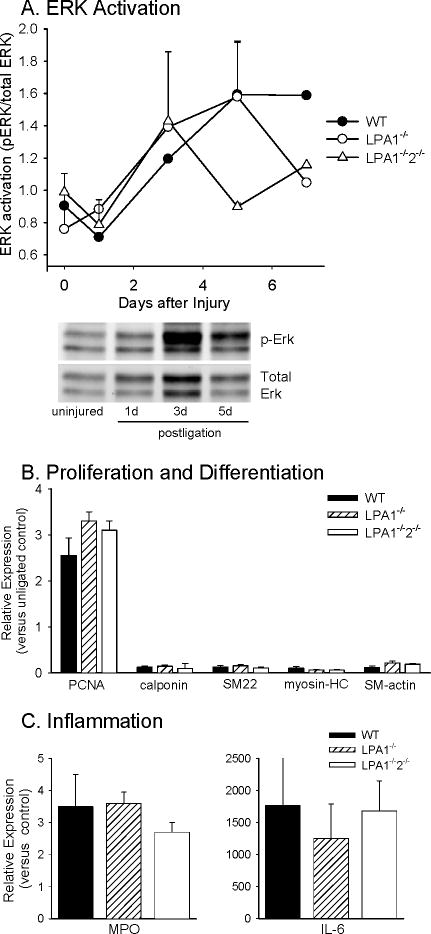
Vascular injury elicits a characteristic sequence of events that includes inflammatory cell recruitment and SMC dedifferentiation, proliferation, and migration. To investigate the mechanism(s) by which LPA receptors might regulate vascular injury responses, we measured markers for these events after ligation. Following injury, there was an increase in ERK activation, as measured by the ratio of phosphoERK to total ERK, and the increase in ERK activity returned to baseline by five days in LPA1-/-2-/- mice and by seven days in LPA1-/- mice (Figure 3A). However, expression of the proliferation marker PCNA was upregulated to similar extents in WT, LPA1-/-and LPA1-/-2-/- vessels (Figure 3B), suggesting that cell proliferation following injury was similar in all genotypes. Likewise, lack of LPA1 and LPA2 did not affect SMC dedifferentiation following injury because down-regulation of SMC markers occurred in all genotypes of mice examined (Figure 3B). Expression of IL-6 and the neutrophil and monocyte derived- inflammatory marker myeloperoxidase (MPO) increased following injury and was also unaffected by the absence of LPA1 and LPA2 (Figure 3C). As detected by immunoblotting, vessel-associated CD68, MPO, and P-selectin, a marker of platelet activation, increased following injury in all genotypes (see Online Figure 2). Taken together, these results indicate that vascular inflammation, SMC dedifferentiation, and cell proliferation are similar in WT, LPA1-/-and LPA1-/-2-/- arteries following injury. Since these markers of proliferation and differentiation were unaltered by combined LPA1 and LPA2 deficiency we considered the possibility that the attenuation of injury induced intimal hyperplasia in LPA1-/-2-/- mice involves a requirement for these receptors as regulators of SMC migration which, as discussed below, we evaluated using primary cultures of mouse SMC.
Figure 3. LPA1 and LPA2 are not required for injury-induced alterations in proliferation, differentiation or inflammation markers.
Carotid injury increases ERK activity in vessels from WT, LPA1-/-, and LPA1-/-2-/- mice (A), although responses in the LPA1-/-2-/- vessels return to baseline within 3 days. Injury upregulates PCNA gene expression and down-regulates SMC differentiation markers in WT (WT), LPA1-/-, and LPA1-/-2-/- vessels (B). Upregulation of myeloperoxidase (MPO) and IL-6 occurs following injury and is similar in WT, LPA1-/-, and LPA1-/-2-/- mice (C). Results are presented as mean ± SD from 3 separate experiments in vessels harvested at five days after injury.
LPA1-/-2-/- vascular SMC exhibit attenuated proliferative responsiveness in vivo and in vitro
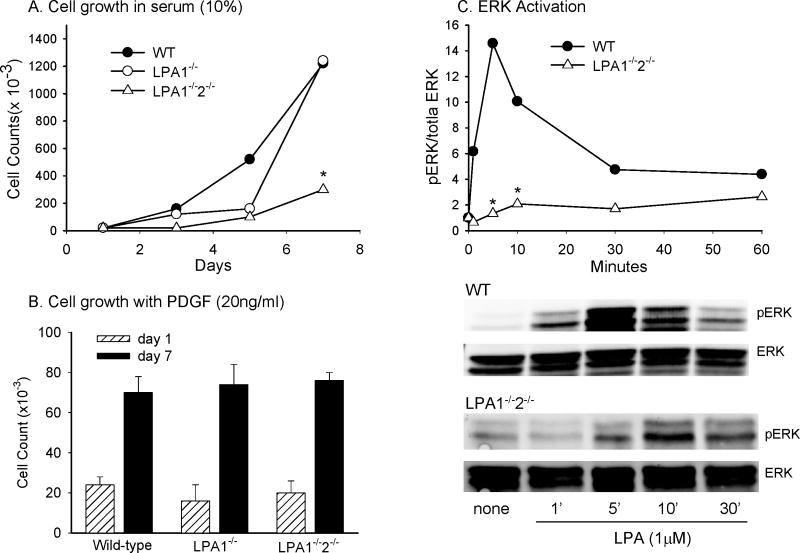
Although LPA responses of cultured SMCs from humans and rats have been investigated in detail these types of studies have not been reported with mouse SMCs. To identify the mechanistic basis for vascular injury response phenotypes observed in the LPA receptor deficient mice, we examined proliferation, dedifferentiation, and migration responses to LPA of isolated mouse vascular SMCs. No significant differences in growth of WT and LPA1-/- SMC in serum were observed, but LPA1-/-2-/- SMC grew more slowly (Figure 4A). This difference appeared to be dependent on the LPA component of serum because there was no difference between PDGF-induced growth of wild type and LPA1-/-2-/- SMC (Figure 4B). LPA regulates cell proliferation through ERK activation in many cell systems. Therefore, we examined LPA-induced ERK responses in isolated SMC. LPA treatment of isolated SMC increased ERK activation maximally at 5 – 10 min (Figure 4C). This response was concentration-dependent with maximal activation observed with 1 μM LPA (data not shown). A similar increase in LPA-induced ERK activity in SMCs cultured from LPA2-/- mice was observed (data not shown). In contrast, there was an approximately two-fold reduction in the initial burst of LPA-induced ERK activation in LPA1-/- SMC and a five-fold reduction in LPA1-/-2-/- SMC (Figure 4C).
Figure 4. Growth and ERK activity is attenuated in LPA1-/-2-/- SMC.
Growth curves for isolated SMC in serum (A) or in response to 20 ng/ml PDGF (B) were generated by counting viable cells at the indicated time points. LPA (1 μM) induces ERK activation in WT cells, as measured by phosphoERK/total ERK ratios. LPA-induced ERK activation is attenuated in LPA1-/-2-/- SMC (C). Results are presented as mean ± SD from three independent cultures of SMC of each genotype. * = p<0.05 in comparison to WT by ANOVA.
LPA1-/-2-/- SMCS exhibit decreased migration in response to LPA, while LPA1-/- SMCs exhibit enhanced migration due to upregulation of the LPA3 receptor
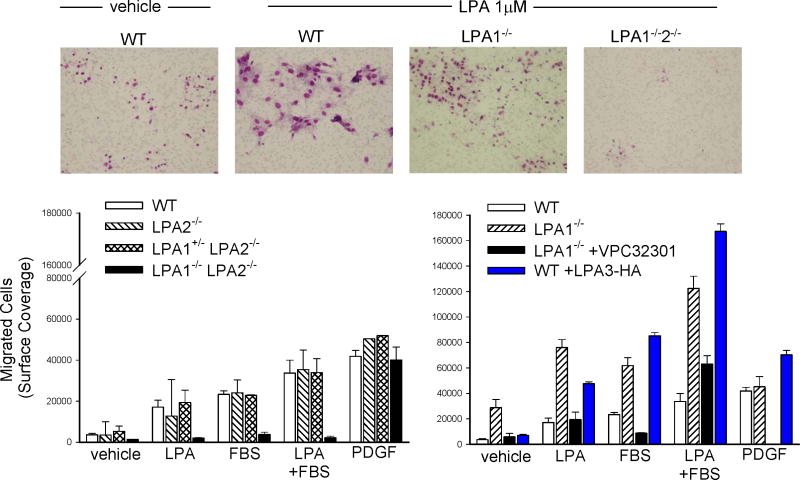
Although the reduced proliferative responses observed in LPA1-/-2-/- SMC in vitro are consistent with the protection of LPA1-/-2-/- mice from the formation of neointima in response to injury, the lack of a change in proliferation markers following injury in LPA1-/-2-/- vessels does not support the idea that protection from the development of intimal hyperplasia is a direct result of attenuated cell proliferation. In many systems, LPA is a potent stimulus for migration, therefore, we compared migration responses to LPA in SMC derived from wild type and LPA receptor deficient mice (Figure 5). LPA, serum, and PDGF stimulated 4.7 ± 0.94, 6.44 ± .046, and 11.5 ± 0.79 fold increases respectively in migration of SMC (Figure 5). The migration of LPA1-/-2-/- cells was lower at baseline (0.39 ± 0.01 that of WT cells; p <0.01), and the cells did not display any increase in migration to LPA and serum (Figure 5) although migration to PDGF was preserved (10.5 ± 1.7 fold increase). In contrast, LPA1-/- cells were hypermigratory at baseline (7.9 ± 1.8 fold higher than WT cells; p = 0.003) and displayed a further exaggerated migration response to LPA and serum (Figure 5).
Figure 5. LPA receptor deficiency alters SMC migratory responses.
SMC were stained with Diff-Quik® on the undersurface of a membrane with a 5 μm pore following migration to media containing vehicle in 0.5% FBS or 1 μM LPA (top). Cell migration in WT, LPA2-/-, LPA1+/-2-/-, and LPA1-/-2-/- cells towards 1 μM LPA, 10% serum, or 20 ng/ml PDGF (bottom left). Comparison of migratory responses of WT and LPA1-/- SMC (bottom right). Enhanced migration in LPA1-/- SMC is attenuated by the LPA3 antagonist VPC32301 (10 μM) and overexpression of LPA3 increases WT migration. Results from at least three separate experiments are reported as mean ± SD of surface coverage by migrated cells in μm2. nd = not determined.
To determine if expression of an alternate LPA receptor might be responsible for the hyper-migratory LPA response observed in the LPA1-/- cells, the assays were conducted in the presence of VPC32301, a selective antagonist of the LPA3 receptor33. LPA3 antagonism had a modest ∼30% inhibition of WT migration (22,978 ± 3507 versus 33,680 ± 6329 μm2 in antagonist-treated and vehicle-treated cells, respectively), and inhibited LPA-induced ERK activation in WT cells by 10 – 15%. LPA3 receptor antagonism reduced both baseline and LPA-induced migration in LPA1-/- cells to levels observed in WT cells (Figure 5) and inhibited LPA-induced ERK activation in LPA1-/- cells by ∼35%. We found that LPA3 receptor expression was elevated 3.7 ± 0.2 fold in LPA1-/-, but not LPA2-/- or LPA1-/-2-/- cells as compared to WT cells (see Online Table 1). To determine if upregulation of LPA3 expression alone could account for the enhanced migration observed in LPA1-/- cells, LPA3 was overexpressed in WT SMC using recombinant lentivirus vectors which resulted in enhanced migration towards LPA and serum phenocopying the behavior of LPA1-/- cells (Figure 5).
LPA1 and LPA2 are not required for Rho activation or SMC dedifferentiation
Migration of SMC to LPA was attenuated by ERK and Rho kinase inhibitors (see Online Figure 3). Therefore, we examined LPA-induced Rho responses in WT, LPA1-/- and LPA1-/-2-/- SMC. In two of three independent SMC cultures, LPA1-/- cells had higher active Rho at baseline, but no differences in LPA-induced Rho activation were observed in WT, LPA1-/- and LPA1-/-2-/- SMC (Figure 6A). These results suggest that lack of ERK activation, rather than changes Rho activation, account for the reduced migration in the LPA1-/-2-/- mice.
Figure 6. Neither LPA1 nor LPA2 are required for isolated SMC dedifferentiation.
Expression of SMC-specific myosin heavy chain in cultured SMCs after serum deprivation, or following re-exposure to either 10% FCS or 1 μM LPA for 24 hours (A). Rho activity was examined 10 min after exposure of cells to LPA (1 μM) by Rhotekin pulldown assay (B). Values represented results obtained from three separate experiments and are reported as a fold change from baseline in WT cells (mean ± SD). S1P = sphingosine-1-phosphate.
Both LPA and Rho have been implicated as regulators of the differentiation state of vascular SMC 3. However, our results following carotid ligation suggest that neither LPA 1 nor LPA2 is required for injury-induced SMC dedifferentiation in vivo. To determine if either of these receptors plays role in dedifferentiation of isolated SMC, we examined expression patterns of the differentiation marker SMC myosin heavy chain. Expression of SMC myosin heavy chain was high in confluent SMC deprived of serum for 72 hours (Figure 6B). Exposure of these cells to 10% serum or 1μM LPA for twenty four hours down-regulated SMC myosin heavy chain expression to a similar extent in WT, LPA1-/- and LPA1-/-2-/- cells (Figure 6B). Taken together, these results suggest that LPA1 and LPA2 are not essential for LPA-triggered dedifferentiation of SMC in vitro.
LPA1 and 2 do not mediate acute blood pressure responses to LPA or regulate systemic blood pressure
The above results suggest that LPA regulates the vascular injury response by modulating migratory properties of SMC. In other species, intravascular administration of LPA alters blood pressure which likely results from direct effects on vascular smooth muscle cell contractility. We sought to determine if the same receptors that regulate vascular SMC migration responses to LPA also contribute to the effects of LPA on blood pressure. Intravenous infusion of BSA conjugated-LPA transiently increased mean arterial blood pressure in anesthetized mice, with 10 pmoles of LPA being the lowest dose that reproducibly increased mean arterial blood pressure above vehicle by 17 ± 6 mm Hg in WT mice. A similar response was observed in mice lacking either LPA1 or LPA2 or both, suggesting that neither receptor is responsible for acute increase in blood pressure elicited by LPA (Table 1). Likewise, there was no difference in blood pressure or heart rate in conscious WT, LPA1-/-, LPA2-/- or LPA1-/-2-/- mice (Table 2).
Table 1.
Blood pressure and heart rate in WT mice and mice lacking LPA1 and/or LPA2
| Genotype | n | Systolic BP* | Heart Rate§ |
|---|---|---|---|
| WT | 6 | 124 ± 26 | 621 ± 38 |
| LPA1-/- | 9 | 126 ± 12 | 621 ± 46 |
| LPA2-/- | 12 | 133 ± 11 | 623 ± 46 |
| LPA1-/-2-/- | 4 | 120 ± 24 | 588 ± 66 |
Values are presented as mean ± SD in mmHg for systolic blood pressure or
beats per minute for heart rate. There is not a statistically significant difference in blood pressure (p = 0.500) or heart rate (p = 0.621).
Table 2.
Increase in mean arterial pressure in response to intravenous administration of LPA
| Genotype | n | Change in MAP* | Weight (gms) |
|---|---|---|---|
| WT | 5 | 17 ± 6 | 29 ± 4 |
| LPA1-/- | 3 | 25 ± 8 | 22 ± 4 |
| LPA2-/- | 3 | 19 ± 8 | 23 ± 1 |
| LPA1-/-2-/- | 2 | 20 ± 2 | 29 ± 4 |
Values are presented as mean ± SD in mmHg. No statisticially significant difference between groups was observed (p = 0.466).
Discussion
In this study, we provide the first demonstration for a role for the LPA signaling nexus in endogenous regulation of pathophysiologic vascular responses. Specifically, we report that mice deficient in two defined LPA receptors, LPA1 and LPA2, are protected from intimal hyperplasia in response to vascular injury through a mechanism that can primarily be accounted for by a decrease in the ability of LPA to promote migration of vascular SMC. Interestingly, we also found that mice lacking LPA1 alone exhibited enhanced vascular injury responses which correlated with an upregulation of a third LPA receptor, LPA3, and appear to result from an enhanced LPA3-dependent vascular smooth muscle cell migratory response. These findings suggest that LPA is a relevant regulator of the murine vascular injury response which focuses attention on possible sources of LPA at sites of vascular injury. We found that injury elevated vessel-associated levels of autotaxin, the lysophospholipase D responsible for generation of extracellular LPA, This may reflect localized expression of autotaxin or, perhaps more likely, recruitment of circulating autotaxin. Because autotaxin is responsible for generating circulating LPA and the autotaxin substrate, lysophosphatidylcholine is abundant in the circulation, the increase in vessel-associated autotaxin likely drives localized production of biologically active, extracellular LPA at the site of injury, which in turn promotes SMC migration via LPA1 and LPA2.
Comparison of the in vivo injury and in vitro SMC responses from mice that are singly or doubly deficient in LPA1 and LPA2 identifies functional redundancy and cross-talk between LPA receptors that has been reported in other cell types including cardiomyocytes and embryonic fibroblasts. For example, LPA-induced ERK activity is normal in LPA2-/- SMCs, slightly reduced in LPA1-/- cells, and nearly completely attenuated in LPA1-/-2-/- SMC, indicating that both LPA1 and LPA2 are required to produce maximal LPA activation of ERK which likely accounts for the reduced proliferative responses in LPA1-/-2-/- cells in vitro. LPA1-/-2-/- SMC display an attenuated migration response to LPA, which may also related to the reduced ERK activity in response to LPA. These results also underscore the importance of the LPA component to the pro-migratory effects of serum on these cells, because LPA1-/-2-/- SMCs display reduced migration to both serum and LPA but not to PDGF.
Our results also point to LPA-dependent vascular signaling pathways that are not regulated by LPA1 or LPA2. Specifically, SMC lacking both receptors appear to undergo essentially normal dedifferentiation in response to serum, LPA, or vascular injury. Because of the possibility that localized production of LPA could promote vasoconstriction at sites of vascular injury we were particularly interested in evaluating the possible roles of LPA1 and LPA2 in blood pressure regulation. However, although these receptors are clearly expressed and functional in vascular SMC, we found that both systemic blood pressure and the acute, transient increase in arterial blood pressure provoked by direct administration of exogenous of LPA was unaltered in LPA1-/-, LPA2-/-, and LPA1-/-2-/- mice.
LPA exerts a range of effects in the cardiovasculature that include modulation of platelet activation, recruitment and activation of inflammatory cells and regulation of blood pressure that likely results from the ability of LPA to promote SMC contractility. Clearly much more work will be required to associate these signaling functions of LPA with the identified LPA receptors. One particularly interesting possibility is that the nuclear receptor PPARγ may mediate some G-protein coupled receptor independent signaling actions of LPA11 and we note that exogenously administered LPA may elicit neointimal formation in rodent vessels in a PPARγ-dependent manner 26.
Emerging evidence supports a role for LPA in regulation of vascular development and function. Mice lacking the LPA synthetic enzyme autotaxin/lysophospholipase D (Enpp2-/-) die embryonically with defects in vascular development 20, 21 and homozygous inactivation of the LPP3 gene which encodes a phosphatase enzyme responsible for inactivation of LPA also results in early embryonic lethality resulting from defects in vascular development and patterning. Our data add to the weight of the evidence that the LPA signaling axis may be important in the regulation of pathophysiologically important vascular cells responses in adult animals. Taken together, our results suggest that interference with the enzymes and receptors responsible for LPA production and signaling would be a viable strategy for pharmacological intervention in the process of intimal hyperplasia.
Supplementary Material
Online Figure 1. Femoral injury was performed in mice as previously described2, 3. Four weeks after injury, vessels were isolated, serially sectioned, and stained with CME. The intimal area was 2.29 ± 0.48 fold greater in LPA1-/- mice (n = 6) as compared to wild-type (WT) animals (n = 7).
Online Figure 2. Changes in inflammatory markers post-injury. CD68 and P-selection expression were measured by immunoblotting at the indicated times post –injury (A). At five days after injury, expression of CD68 and P-selection was measured in WT, LPA1-/-, and LPA1-/-2-/- carotids (B). Myeloperoxidase (MPO) activity in the vessel was measured at the indicated times as previously described4 (C). nd = not detemined.
Online Figure 3. SMC migration to LPA is attenuated by ERK and Rho kinase inhibitors. SMC migration was performed as described in Online Supplemental Methods in the presence of the ERK inhibitor PD98059 (10 μM) or the Rho kinase inhibitor Y-27632 (10 μM).
Online Table 1. LPA receptor expression in LPA1-/-, LPA2-/-, and LPA1-/-2-/- SMC relative to WT SMC
Acknowledgments
The authors wish to thank Jessica Caicedo, Kirk McNaughton and Raina Tosheva for excellent technical assistance. This work was supported by NIH grants HL070304, HL078663, and HL074219 to S.S.S., CA096496 and GM050388 to A.J.M and MH51699 and NS048478 to J.C.. S.S.S. received an Atorvastatin Research Award from Pfizer.
Footnotes
Disclosures: None.
Reference List
- 1.Kawai-Kowase K, Owens GK. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C59–C69. doi: 10.1152/ajpcell.00394.2006. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, Aoki J, Arai H, Sobue K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–8. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 4.Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendis MR, Dhalla NS. Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol. 2003;19:1525–36. [PubMed] [Google Scholar]
- 5.Siess W, Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem. 2004;92:1086–94. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 6.Tokumura A, Iimori M, Nishioka Y, Kitahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. Am J Physiol. 1994;267:C204–C210. doi: 10.1152/ajpcell.1994.267.1.C204. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz U, Thommes K, Beier I, Vetter H. Lysophosphatidic acid stimulates p21-activated kinase in vascular smooth muscle cells. Biochem Biophys Res Commun. 2002;291:687–91. doi: 10.1006/bbrc.2002.6493. [DOI] [PubMed] [Google Scholar]
- 8.Gennero I, Xuereb JM, Simon MF, Girolami JP, Bascands JL, Chap H, Boneu B, Sie P. Effects of lysophosphatidic acid on proliferation and cytosolic Ca++ of human adult vascular smooth muscle cells in culture. Thromb Res. 1999;94:317–26. doi: 10.1016/s0049-3848(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 9.Damirin A, Tomura H, Komachi M, Liu JP, Mogi C, Tobo M, Wang JQ, Kimura T, Kuwabara A, Yamazaki Y, Ohta H, Im DS, Sato K, Okajima F. Role of lipoprotein-associated lysophospholipids in migratory activity of coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2513–H2522. doi: 10.1152/ajpheart.00865.2006. [DOI] [PubMed] [Google Scholar]
- 10.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre TM, Pontsler AV, Silva AR, St HA, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–6. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaits F, Fourcade O, Le BF, Gueguen G, Gaige B, Gassama-Diagne A, Fauvel J, Salles JP, Mauco G, Simon MF, Chap H. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 1997;410:54–8. doi: 10.1016/s0014-5793(97)00411-0. [DOI] [PubMed] [Google Scholar]
- 13.Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989;1001:282–5. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 14.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 15.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986;875:31–8. [PubMed] [Google Scholar]
- 16.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–25. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277:48737–44. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 18.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–80. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 21.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federico L, Pamuklar Z, Liu S, Goto M, Dong A, Berdyshev E, Natarajan V, Fang F, Mills GB, Morris AJ, Smyth SS. Overexpression of autotoxin/lysophospholipase D reveals a novel path for regulation of hemostasis in mice. Blood. 2007;110:3649. [Google Scholar]
- 23.Tokumura A, Fukuzawa K, Tsukatani H. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids. 1978;13:572–4. doi: 10.1007/BF02533598. [DOI] [PubMed] [Google Scholar]
- 24.Tigyi G, Hong L, Yakubu M, Parfenova H, Shibata M, Leffler CW. Lysophosphatidic acid alters cerebrovascular reactivity in piglets. Am J Physiol. 1995;268:H2048–H2055. doi: 10.1152/ajpheart.1995.268.5.H2048. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, Arai H, Sobue K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108:1746–52. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–74. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–9. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–9. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar PP, Good RR, Linder J. Complete response of granulosa cell tumor metastatic to liver after hepatic irradiation: a case report. Obstet Gynecol. 1986;67:95S–8S. doi: 10.1097/00006250-198603001-00028. [DOI] [PubMed] [Google Scholar]
- 30.Myers DL, Liaw L. Improved analysis of the vascular response to arterial ligation using a multivariate approach. Am J Pathol. 2004;164:43–8. doi: 10.1016/S0002-9440(10)63094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P, Patil S, Rojas M, Fong AM, Smyth SS, Patel DD. CX3CR1 deficiency confers protection from intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2006;26:2056–62. doi: 10.1161/01.ATV.0000234947.47788.8c. [DOI] [PubMed] [Google Scholar]
- 32.Smyth SS, Reis ED, Zhang W, Fallon JT, Gordon RE, Coller BS. Beta(3)-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–7. doi: 10.1161/01.cir.103.20.2501. [DOI] [PubMed] [Google Scholar]
- 33.Heasley BH, Jarosz R, Carter KM, Van SJ, Lynch KR, Macdonald TL. A novel series of 2-pyridyl-containing compounds as lysophosphatidic acid receptor antagonists: development of a nonhydrolyzable LPA3 receptor-selective antagonist. Bioorg Med Chem Lett. 2004;14:4069–74. doi: 10.1016/j.bmcl.2004.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure 1. Femoral injury was performed in mice as previously described2, 3. Four weeks after injury, vessels were isolated, serially sectioned, and stained with CME. The intimal area was 2.29 ± 0.48 fold greater in LPA1-/- mice (n = 6) as compared to wild-type (WT) animals (n = 7).
Online Figure 2. Changes in inflammatory markers post-injury. CD68 and P-selection expression were measured by immunoblotting at the indicated times post –injury (A). At five days after injury, expression of CD68 and P-selection was measured in WT, LPA1-/-, and LPA1-/-2-/- carotids (B). Myeloperoxidase (MPO) activity in the vessel was measured at the indicated times as previously described4 (C). nd = not detemined.
Online Figure 3. SMC migration to LPA is attenuated by ERK and Rho kinase inhibitors. SMC migration was performed as described in Online Supplemental Methods in the presence of the ERK inhibitor PD98059 (10 μM) or the Rho kinase inhibitor Y-27632 (10 μM).
Online Table 1. LPA receptor expression in LPA1-/-, LPA2-/-, and LPA1-/-2-/- SMC relative to WT SMC