Abstract
Oxytocin regulates partner preference formation and alloparental behavior in the socially monogamous prairie vole (Microtus ochrogaster) by activating oxytocin receptors in the nucleus accumbens of females. Mating facilitates partner preference formation, and oxytocin-immunoreactive fibers in the nucleus accumbens have been described in prairie voles. However, there has been no direct evidence of oxytocin release in the nucleus accumbens during sociosexual interactions, and the origin of the oxytocin fibers is unknown. Here we show for the first time that extracellular concentrations of oxytocin are increased in the nucleus accumbens of female prairie vole during unrestricted interactions with a male. We further show that the distribution of oxytocin-immunoreactive fibers in the nucleus accumbens is conserved in prairie voles, mice and rats, despite remarkable species differences in oxytocin receptor binding in the region. Using a combination of site-specific and peripheral infusions of the retrograde tracer, Fluorogold, we demonstrate that the nucleus accumbens oxytocin-immunoreactive fibers likely originate from paraventricular and supraoptic hypothalamic neurons. This distribution of retrogradely labeled neurons is consistent with the hypothesis that striatal oxytocin fibers arise from collaterals of magnocellular neurons of the neurohypophysial system. If correct, this may serve to coordinate peripheral and central release of oxytocin with appropriate behavioral responses associated with reproduction, including pair bonding after mating, and maternal responsiveness following parturition and during lactation.
Keywords: pair bonding, nucleus accumbens, paraventricular nucleus, supraoptic nucleus, neurohypophysial peptides, alloparental behavior
INTRODUCTION
Oxytocin (OT) released from the neurohypophysial system has been implicated in the regulation of reproductive physiology in mammals, including uterine contractions during parturition and milk ejection during lactation (Burbach et al., 2006). In addition, OT released within the brain coordinates the onset of maternal responsiveness and maternal bonding at the time of parturition (Pedersen and Prange, 1979, Kendrick et al., 1987). Recent studies in humans have also suggested that central OT modulates social cognition, including increasing interpersonal trust, eye gaze, face recognition, and the ability to infer the emotions of others based on facial cues (Kosfeld et al., 2005, Domes et al., 2007, Donaldson and Young, 2008, Guastella et al., 2008, Savaskan et al., 2008).
Prairie voles (Microtus ochrogaster) have become an important animal model for elucidating the behavioral roles of OT and the neurobiology of affiliative behavior (Carter et al., 1995, Young and Wang, 2004). Prairie voles are a highly affiliative rodent species characterized by a socially monogamous mating strategy and high levels of alloparental care. In the laboratory, the formation of selective pair bonds between mates can be assessed using a partner preference test in which the time spent with the partner versus a novel stimulus animal is quantified. Extended cohabitation with a male or mating facilitates the formation of partner preferences in female prairie voles (Williams et al., 1992). Pharmacological and genetic manipulation studies have demonstrated that oxytocin receptors (OTR) in the nucleus accumbens (NAcc) play a significant role in the regulation of behaviors associated with social monogamy and alloparental care. Infusion of an OTR antagonist into the NAcc prevents mating-induced partner preferences in female prairie voles (Young et al., 2001). Conversely, increasing OTR density in the NAcc using viral vector gene transfer can accelerate the formation of a partner preference (Ross et al., 2009b). Furthermore, OTR binding density in the NAcc is positively correlated with alloparental behavior, and infusion of an OTR antagonist into the NAcc inhibits spontaneous alloparental behavior in sexually naïve female prairie voles (Olazabal and Young, 2006a). Interestingly, non-monogamous rodent species, including meadow voles, mice and rats have very low levels of OTR binding in the NAcc, which may contribute to the species differences in social behavior (Insel and Young, 2001, Burbach et al., 2006).
Despite the evidence that OT signaling in the NAcc plays a critical role in regulating affiliative behavior in prairie voles, the presynaptic OT system in this region has not been characterized. Specifically, mating-induced OT release has not been directly demonstrated, and the morphology and source of the OT-immunoreactive fibers projecting to the NAcc has not been determined. OT is produced primarily in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus. Large diameter magnocellular neurons in these regions form the neurohypophysial system and are thought to project primarily to the posterior pituitary (Bargmann, 1949). Early tract tracing studies demonstrated that OT-immunoreactive fibers of the brainstem originate from the smaller diameter parvocellular OT neurons in the PVN, leading to the assumption that these neurons also project to the forebrain regions regulating behavior, providing a dissociation between the neurohypophysial and central OT systems (for review see (Landgraf and Neumann, 2004).
In this study we examined the NAcc OT system in detail. Using microdialysis in awake, behaving female prairie voles, we measured OT release within the NAcc as a function of sociosexual interactions with a male. We then compared the distribution of OT-immunoreactive fibers in the NAcc of prairie voles, meadow voles, rats and mice to determine whether the OT innervation of the NAcc is conserved across species with diverse social behavior and OTR distributions in this area. The ultrastructural features of the OT-immunoreactive processes in the NAcc were then examined using electron microscopy. Finally, Fluorogold tract tracing was used to identify the neuronal origin of the OT projections to the NAcc. The results of these studies add significantly to our understanding of the circuitry involved in regulating affiliative behavior in female prairie voles, and provide a potential mechanism for coordinating central OT release with reproductive physiology in all mammals.
EXPERIMENTAL PROCEDURES
Animals
Prairie and meadow voles were housed in same sex groups with 2–3 voles/cage from the time of weaning at 21–23 days of age. Housing consisted of a ventilated 36×18×19cm Plexiglass cage filled with Bed-o-cobbs Laboratory Animal Bedding under a 14:10 hr light/dark cycle at 22°C with access to food (rabbit LabDiet, Richmond, IN) and water ad libitum. The prairie voles were obtained from our laboratory breeding colony that originally derived from field-captured voles in Illinois. Meadow voles originated from a colony at Florida State University. For the microdialysis experiment, subjects were sexually naïve female prairie voles 70–90 days of age (30–45g).
For anatomical studies, subjects were adult (>60days old) female prairie voles. In addition, five female sexually naïve meadow voles from our breeding colony, five virgin female mice (C57BL/6J), and five virgin Sprague Dawley (Charles River) female rats were used for a species comparison. All procedures were approved by the Emory University Institutional Animal Care and Use Committee.
Hormone Treatment and Microdialysis Probe Implantation
Subjects were ovariectomized two weeks prior to probe implantation and microdialysis, and administered 1µg estradiol benzoate in peanut oil, intraperitoneally (IP), for four consecutive days prior to testing to induce receptivity. Subjects were anesthetized with isoflurane and U-shaped microdialysis probes were stereotaxically implanted unilaterally into the NAcc (nose bar −2.5mm, AP +1.8mm, ML −0.9mm, DV −4.5mm). After a one day recovery, the probe was connected to two lengths of PE20 tubing (Polymicro Tech, Phoenix, AZ). The microdialysis probes were self made as previously described, and had a molecular cut-off of 18 kDa (for details see Neumann et al., 1993; Bosch et al., 2005). The inlet of the probe was connected to a 10-ml Hamilton syringe controlled by a CMA/100 microinfusion pump (Bioanalytical Systems, West Lafayette, IN). The outlet fed into a refrigerated collector (SciPro, Inc, Sanborn, NY) housing polyethylene tubes (Fisher Scientific, Houston, TX). A single-channel swivel and counter-balanced lever arm allowed the animals to move freely during mating. Ringer’s solution was perfused through the probe via the inlet into the NAcc during the experiment at a flow rate of 1.0µl/min.
Oxytocin Collection
To determine the effects of sociosexual interactions with a male on OT release, eight consecutive 30-minute microdialysates were collected from the NAcc of estrogen-primed female prairie voles (n=26). Sample collections were divided into three phases: basal (1–4), restricted exposure (5–8), and free exposure (9–16). Basal dialysates were collected from individually housed females and served as the baseline for extracellular concentration of OT. After the basal phase, a sexually experienced male prairie vole of similar age and weight, housed in a wire mesh cage (restricted exposure phase), was introduced into the test cage. After 2 hrs the male was removed from the mesh cage and allowed to physically interact with the female (free exposure phase) for 4 h. This portion of the test was videotaped. Female subjects that did not mate during this period because they were not sexually receptive were categorized as non-mated. Following microdialysis, brains were rapidly removed, frozen on dry ice, and stored at −80°C until use. Brains were later sectioned on a cryostat into 20-µm slices mounted on Superfrost plus microscope slides (Fisher, Pittsburgh, PA). Slides were stored at −80°C. Proper placement of the probes was confirmed by cresyl violet staining.
Quantification of OT
Dialysates collected during microdialysis were stored at −80°C until analyzed for content. Samples were lyophilized and the concentration of OT in each dialysate was determined by radioimmunoassay as described previously (Neumann et al., 1993). Cross-reactivity of the polyclonal antiserum with arginine-vasopressin and other related peptides was <0.7%. Intra- and inter-assay coefficients of variation were in the 5–9% and 8–12% ranges, respectively; all dialysates to be compared were assayed in the same run. 25 µl of each dialysate was assayed and the level of detectability of the assay was 0.05pg/dialysate.
Fluorogold Infusions
Female prairie voles (n=18) were anesthetized with isoflurane and placed in a Kopf stereotaxic apparatus. Fluorogold (FG) was iontophoretically injected unilaterally into the NAcc (AP +1.7mm, ML −0.9mm, DV −4.5mm) using a glass micropipette (tip diameter 10– 20 µM) filled with the retrograde tracer Fluorogold (2–4% soln. w/v in dH20; Fluorochrome LLC; Denver, CO) for 15 min at 1–7 µA (50/50 duty cycle). The syringe was left in place for 5 min following infusion to minimize diffusion of tracer up the needle track. 7–14 days after injection, the animals were perfused as described below. Animals with injections that did not reach the NAcc, core or shell, contaminated the lateral ventricle, or showed no FG staining at the injection site were not included in the analysis. To control for the possibility that leakage of FG into capillaries near the injection site or into the ventricles resulted in labeling of hypothalamic neurons, we also performed control infusions consisting of an injection of 0.01 µg of FG in 500nl dH2O into the lateral ventricle, or 0.5 µg of FG in 50 µl dH2O injected IP. These control animals were perfused a week after injection, as described below.
In order to label neurons of the neurohypophysial OT system that send projections to the pituitary, a region in the CNS that lacks a blood-brain barrier (Merchenthaler, 1991), 600 µg of FG (Horvath, 1998) in 75 µl dH2O was injected IP into female voles. Five days later, the tissue was collected as described below.
Tissue Collection for Immunohistochemistry
Animals were euthanized and perfused transcardially with 50 ml of PBS, followed by 50 ml of 4% paraformaldehyde in 0.1 M phosphate buffer containing either 0.1% glutaraldehyde for EM or 2.5% acrolein (Polysciences, Warrington, PA) for all other anatomical studies. Immediately following perfusion, the brains were removed and stored at 4°C in 30% sucrose solution until sectioned. The brains were cut into 25 µm coronal or 60 µm horizontal sections with a freezing microtome, or 60 µm coronal sections with a vibratome, and stored free-floating in cryoprotectant solution at −20°C until immunocytochemical processing.
Single labeling immunohistochemistry for OT or FG
A 1:6 series through each brain was processed for FG and/or OT for either electron (EM), light (LM), or fluorescent (FM) microscopy. Briefly, sections were removed from the cryoprotectant solution, rinsed extensively in potassium phosphate-buffered saline (KPBS; pH 7.4), and then reacted for 15 minutes in 1% sodium borohydride to remove excess aldehydes. Sections were then incubated in primary antibody solution directed against either FG or OT in KPBS containing 0.1% Triton-X for 48 hours at 4°C. Cells containing OT were identified by using a polyclonal rabbit anti-OT antibody (20068; Immunostar, Hudson, WI) (Lim et al., 2004) at a concentration of 1:1000 for EM, 1:70,000 for LM and 1:10,000 for FM. Cells containing FG were identified by using the polyclonal rabbit anti-Fluoro-Gold antibody (AB153;Chemicon, Temecula, CA) at a concentration of 1:5000. After primary antibody incubation, the tissue was rinsed in KPBS, incubated for 1 hour in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) at a concentration of 1:600 for LM and 1:5000 for FM, and rinsed in KPBS, followed by a 1-hour incubation in avidin-biotin peroxidase complex (Vector, Burlingame, CA, ABC Elite Kit PK-6100) at a concentration of 1:100 for LM and 1:450 for FM. At this point, single labeled tissue for LM was rinsed in either 1) KPBS and sodium acetate (0.175 M; pH 6.5), and visualized as a black reaction product by using nickel sulfate-intensified 3,3′-diaminobenzidine solution containing 0.08% hydrogen peroxide in sodium acetate buffer, or 2) KPBS and Tris buffer (pH 7.2), and visualized as a brown reaction product by using 3,3′-diaminobenzidine (DAB) containing 0.08% hydrogen peroxide in Tris buffer (pH 7.2). The reaction product was terminated after approximately 20 minutes by rinsing in sodium acetate buffer or Tris buffer, respectively. Double labeled tissue for FM continued to be processed as described below.
Double Label Tyramine Amplification Protocol
After rinsing FG-labeled tissue out of the ABC solution (see above), the tissue was incubated in biotin-tyramine (BT) solution (5ul BT/ml KPBS) for 20 minutes, rinsed in KPBS, incubated with Texas Red-streptavidin (T02,Biomeda, Foster City,CA) or stretavidin Alexa Fluor 350 (S11249; Invitrogen) in heated KPBS containing 0.4% Triton-X-100 for 3 hours at 37°C at a concentration of 1:200. Slides were then rinsed in KPBS, incubated overnight in anti-OT antibody solution in KPBS containing 0.1% Triton-X-100 and normal goat serum (Jackson Immunoresearch, West Grove, PA) (1:100) at room temperature, rinsed in KPBS, incubated in Cy2 conjugated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) (1:50) and normal goat serum (1:100) for 2.5 hours, and then rinsed in KPBS. All sections were mounted out of saline onto gelatin-subbed slides, air-dried overnight, dehydrated in a series of graded alcohols, cleared in Xylene, and coverslipped using Krystalon (EMD Chemicals, Gibbstown, NJ). The tyramine amplification method allows for the use of primary antibodies raised in the same species to be used for double labeling immunohistochemistry without cross-reactivity (Shindler and Roth, 1996, Guidetti et al., 2007).
Single Pre-embedding Immunoperoxidase Labeling for EM
Following sodium borohydride treatment (see above), sections were placed in a cryoprotectant solution (phostphate buffer (PB) 0.05M, pH 7.4, 25% sucrose, and 10% glycerol) for 20 minutes, frozen at −80°C for 20 minutes, returned to a decreasing gradient of cryoprotectant solutions, and rinsed in PBS. Sections were then incubated in primary and secondary antibody solutions identical to those used for LM (see above), except for the omission of Triton X-100.
After the DAB reaction, the tissue was rinsed in PB (0.1M, pH 7.4) and treated with 1% OsO4 for 20 minutes. It was then returned to PB and dehydrated with increasing concentrations of ethanol. When exposed to 70% ETOH, 1% uranyl acetate was added to the solution for 35 minutes to increase the contrast of the tissue at the electron microscope. Following dehydration, sections were treated with propylene oxide and embedded in epoxy resin for 12 hours (Durcupan ACM, Fluka, Buchs, Switzerland), mounted onto slides and placed in a 60°C oven for 48 hours. Separate samples of the NAcc were cut out of the larger sections, mounted onto resin blocks and cut into 60 nm sections using an ultramicrotome (Leica Ultracut T2). The 60 nm sections were collected on Pioloform-coated copper grids, stained with lead citrate for 5 minutes to enhance tissue contrast and examined on the Zeiss EM-10C electron microscope. Electron micrographs were taken with a CCD camera (DualView 300W; Gatan, Inc., Pleasanton, CA) controlled by DigitalMicrograph software (Gatan, Inc.).
Semi-Quantitative Analysis of Accumbal Oxytocin Fibers Across Species
The density of OT-immunoreactive fibers was semi-quantitatively compared across four species: prairie vole, meadow vole, rat and mouse. Subjects in this study were all sexually naïve adult females (N=5 females/species). Prairie and meadow voles display induced estrus, and were therefore reproductively quiescent. We did not control for the estrus cycle in the rats or mice. All tissue in this study was processed in a single assay. Three sections through the NAcc were bilaterally analyzed for OT fiber density using the AIS 6.0 image analysis system (MCID, Canada). Images were captured using a 10x objective and the threshold was adjusted to distinguish immunoreactive elements from the background. The percentage of total field pixels covered by immunoreactive elements was calculated by the software. Data from all measurements in a single animal were averaged to yield a single data point per animal.
Quantification of Retrograde Labeling
The number of FG+, OT+, and FG+OT+ cells were manually counted in each section throughout the extent of the PVN using fluorescent microscopy. Quantification was only performed on the subset of animals displaying double-labeled FG+OT+ cells. Cells were considered FG+_only if the cell body contained clear fluorescent punta.
Statistical Analysis
The level of detectability for OT was 0.05pg/dialysate. As most samples were below the level of detectability for OT we used a binary categorization—detectable or non-detectable—for each dialysate. Fisher’s exact test was used to compare the frequency of detectable OT between phases and groups. A Kruskal-Wallis One Way Analysis of Variance on Ranks was used to determine whether fiber density in the NAcc (expressed as proportional area) varied across species. Data are presented as mean ± standard error of the mean (SEM).
RESULTS
OT Release During Sociosexual Interactions
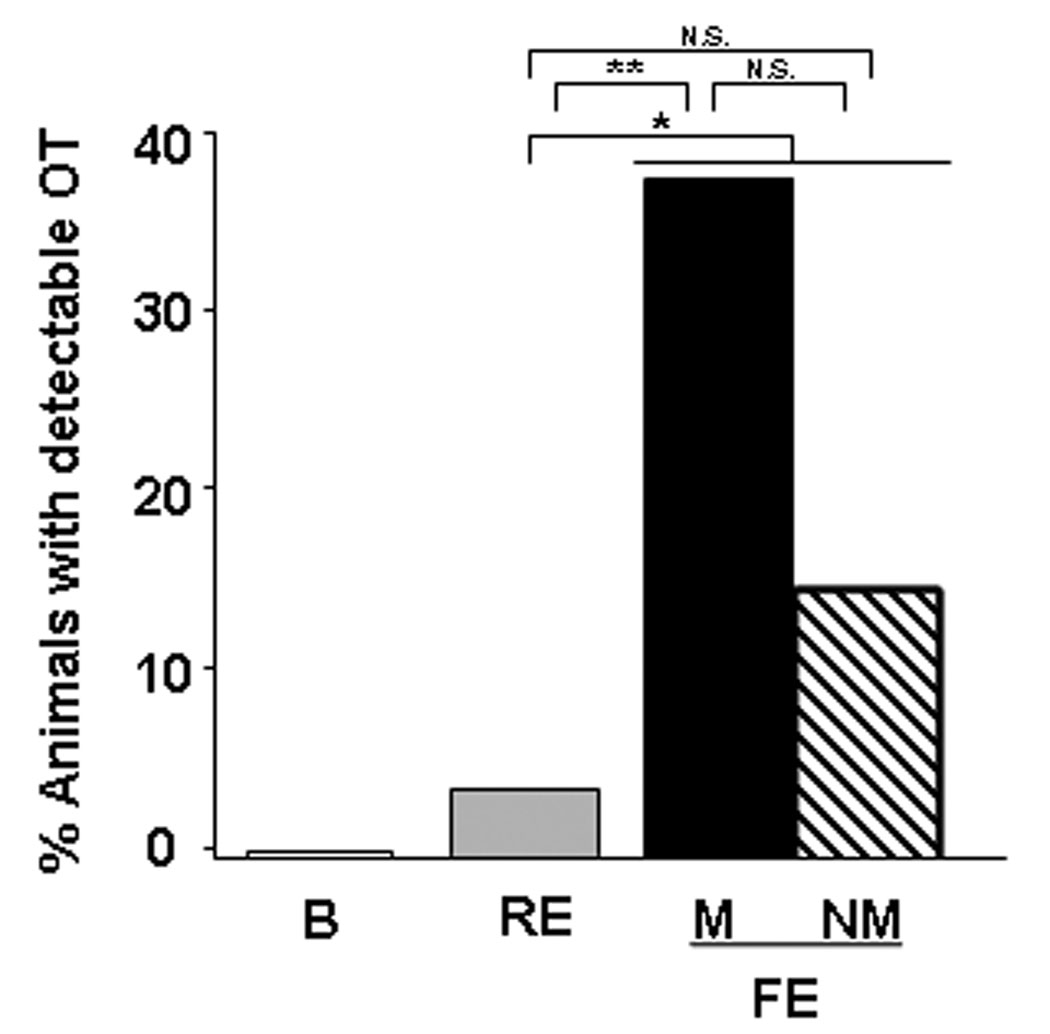
In vivo microdialysis was performed in female prairie voles at baseline, during a restricted exposure when the male was confined to a wire cage, and during free exposure when mating could occur. OT concentrations in the dialysates were below the level of detectability in the majority of samples. Therefore, samples were categorized as being detectable (> 0.05 pg/25 µl sample) or non-detectable (< 0.05 pg/25 µl sample) and analyzed using the non-parametric Fisher’s exact test. None of the animals produced microdialysate samples with detectable OT during the basal phase. Overall, the number of animals producing microdialysate with detectable OT was greater during the free exposure phase compared to the restricted exposure phase (Fisher’s exact test p = 0.05; Figure 1). Furthermore, when the group was split based on whether mating occurred in the free exposure phase, the number of animals having detectable OT was greater in the free exposure phase than in the restricted exposure phase for those animals that mated (N=13, Fisher’s exact test, P = 0.039; Figure 1), but this was not the case for the group of animals who failed to copulate during the free exposure phase (N=13, Fisher’s exact test, p = > 0.5; Figure 1). However, there was no statistical difference between the number of animals showing detectable OT during the free exposure phase between the groups that mated vs those that did not mated (Fisher’s exact test, p = 0.38; Figure 1). It should be noted that animals that mated received multiple intromissions throughout the free exposure phase, while the animals that did not mate received attempted mounts from the males.
Figure 1.
In vivo microdialysis to detect extracellular oxytocin (OT) as a function of social exposure and mating. Four 30-min samples were collected and analyzed for each phase. The graph illustrates the percentage of animals yielding microdialysates in each phase with detectable OT concentrations. Under basal conditions (B) OT concentrations were below the level of detectability (<0.05 pg/sample) in all samples. Detectable OT was observed significantly more frequently during the free exposure (FE) phase compared to during the restricted exposure (RE) phase when the male was housed in a wire cage (* = Fisher’s exact test, P = 0.05). In addition, detectable OT was observed significantly more frequently during the FE phase in females that mated (M) compared to during the RE (** = Fisher’s exact test, P = 0.039). In the group of females that failed to mate (NM) during the free exposure phase, the percentage with detectable OT during that phase was not significantly different from the restricted exposure phase (Fisher’s exact test, P > 0.05). There was no significant difference in the number of females that mated vs unmated during the FE phase (Fisher’s exact test, P > 0.05).
Species Comparisons of OT Immunoreactivity in the NAcc
The distribution of OTR in the NAcc is highly species specific, with prairie voles having high densities of OTR, rats having intermediate densities of OTR, and mice and meadow voles having little or no OTR (Burbach et al., 2006, Ross and Young, 2009). By contrast, OT peptide expression is highly conserved across these species. We examined OT fiber-immunoreactivity in the NAcc of prairie voles, meadow voles, mice and rats. The distribution of OT-immunoreactive fibers was qualitatively similar across species, with sparse fibers in the anterior NAcc, primarily in the shell, which in more caudal regions become denser at the ventral pallidum and diagonal band (Figure 2). A semiquantitative analysis of the density of OT-immunoreactive fibers in the NAcc revealed no statistically significant difference in the proportion of area covered by OT immunoreactive fibers across species (prairie vole: 3.3 ± 0.47%, meadow vole: 4.2 ± 0.69%, mouse: 3.0 ± 0.13%, rat: 3.0% ± 0.40%) (H=3.50, p > 0.05).
Figure 2.
Species comparison of OT immunoreactive fibers in coronal sections of the nucleus accumbens (NAcc). Prairie voles (A), meadow voles (B), mice (C) and rats (D) all displayed a comparable pattern of distribution and relative density of OT-immunoreactive fibers in the NAcc. Scale bar = 500 µm (valid for A–D). ac = anterior commisure, PVN = paraventricular nucleus.
Morphological Characteristics of NAcc OT Processes
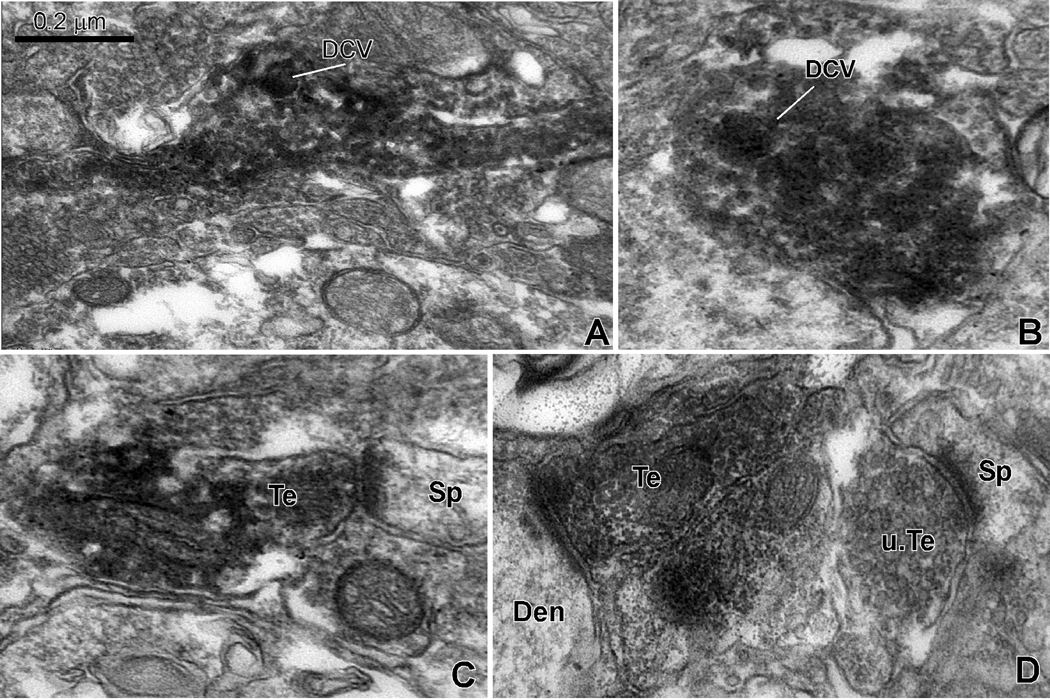
Electron microscopy was used to characterize the ultrastructure of the OT-immunoreactive processes in the NAcc. Two major types of OT-immunoreactive elements were found in NAcc: (1) Large caliber unmyelinated structures filled with OT-immunoreactive dense core vesicles that do not form clear synaptic contacts but are often apposed to dendrites of striatal neurons (Figure 3A,B). (2) Vesicle-filled synaptic terminals that form clear asymmetric synapses most commonly with spine heads and less frequently with dendritic shafts of striatal neurons (Figure 3C,D). The latter type of terminals displayed ultrastructural features of glutamatergic boutons and were most often devoid of OT-immunoreactive dense core vesicles. Random analysis of all labeled structures seen in this material revealed that the non-synaptic dense core vesicle filled structures accounted for 73% (32 out of 44) of total labeled elements examined, whereas OT-positive terminals represented only 23% (12 out of 44) of total labeled structures. Of these 12 OT-positive terminals, 83% (10 out of 12) formed asymmetric synaptic contact with spines, while 17% (2 out of 12) were in contact with dendritic shafts.
Figure 3.
Electron micrographs of OT-immunoreactive elements on the nucleus accumbens. (A and B) show strongly labeled profiles that contains darkly stained dense core vesicles (DCV). These elements did not form clear synaptic contact in the planes of sections examined. (C and D) illustrate examples of OT-immunoreactive terminal boutons (Te) forming asymmetric synapses with a spine (sp) and a dendritic shaft (Den). An unlabeled terminal forming an asymmetric axo-spinous synapse is shown in D. Scale bar in A valid for B–D.
Visualization of OT Fiber Projections in Horizontal Sections
To delineate the course of OT-immunoreative fibers to the NAcc, horizontal sections through the NAcc and PVN were stained for OT. Figure 4 illustrates that the OT-immunoreactive fibers traversing toward the NAcc often emerge from, and are indistinguishable from, the dense hypothalamic fiber tracts of the neurohypophysial system. Some of these fibers seem to deviate from the other fibers and travel rostrally towards the striatum. This view also illustrates the diffuse pattern of OT-immunoreactive throughout the forebrain and striatum.
Figure 4.
Light micrograph of oxytocin-immunoreactive fibers in the paraventricular nucleus of the hypothalamus (PVN) and nucleus accumbens (NAcc) of the prairie vole from a horizontal section. Black line delineates the boundary of the striatum as determined by a marker for KChIP2. Note the diffuse pattern of immunoreactive fibers coursing toward the striatum. A few fibers deviate from the neurohypophysial pathway of the PVN and project toward the striatum. Scale bar = 100 µm. ac = anterior commisure, f = fornix.
Identification of the OT Neurons Projecting to the NAcc
The retrograde tracer FG was used to determine the origin of OT neurons that project to the NAcc. The FG infusions targeted the shell of the NAcc; the same region of the microdialysis probe placement, and the location of the OT-immunoreactive fibers. Examination of the FG injection halo by immunohistochemistry revealed that 11 of the 18 animals had FG halos in the shell and included portions of the medial core of the NAcc. The FG halo of two of these animals also included the medial and dorsal core, and one included the shell only. An additional animal had an injection that included the shell and portions of the lateral septum and was therefore removed from analysis. Thus 10 animals had FG injection sites that met the inclusion criteria (Figure 5A). One of the strongest projections to the NAcc originated from the midline thalamic cell groups, including the reuniens nucleus, xiphoid nucleus, parataenial nucleus, and medial dorsal nucleus (Figure 5B). Other FG-labeled areas included the prelimbic cortex, ventral pallidum, lateral septum, anterior amygdala, and ventral tegmental area. FG labeling in these areas was consistent across all of the animals. In addition, double labeled (FG-positive and OT-positive) cells were present in the PVN (Figure 5C,D) and, to a lesser extent, in the SON (Figure 5E,F). A 1:6 series yielded 3–5 double labeled cells in the PVN of 6 of the 10 females, while two animals had a single SON double-labeled soma. Counts through the PVN of these animals showed there was an average of 20.5 FG cells and 433.8 OT cells in the 1:6 series. In addition, 23% of FG cells contained OT, while 0.7% of OT cells contained FG. Since projections from the PVN and SON to the NAcc have not been previously reported, control injections into the lateral ventricles and periphery were performed that used similar amounts of FG to what was injected into the NAcc. The peripheral IP infusion (0.5 µg/vole) was performed because FG in the bloodstream is taken up by hypothalamic terminals in the pituitary. No FG-labeled neurons were detected in the PVN or SON of any of the control animals. This suggests that the double-labeled neurons in the NAcc-infused brains represent bona fide projections from these nuclei to the NAcc.
Figure 5.
Retrograde labeling from the nucleus accumbens (NAcc) of female prairie voles. A) Representative injection site of Fluorogold (FG) in the NAcc. B) FG+ cells in the medial dorsal (MD) and paratenial (PT) thalamic nuclei, one of the most heavily stained regions after these NAcc injections. C–D) Arrows indicate double labeled neurons for FG (C) and OT (D) in the paraventricular nucleus of the hypothalamus. E–F) The arrows indicates a double labeled neurons for FG (E) and OT (F) in the supraoptic nucleus of the hypothalamus. Scale bars: A = 200 µm; B = 100 µm; F = 100 µm (valid for C–E). LV = lateral ventricle, ac = anterior commisure, 3V = third ventricle.
Organization of the Pituitary Projecting OT System
Peripherally injected retrograde tracer can be taken up by cells that have terminals outside the blood brain barrier, including the magnocellular neurons projecting to the posterior pituitary and the parvocellular neurons projecting to the median eminence. Thus, OT cells labeled with FG following a peripheral injection represent magnocellular neurons of the neurohypophysial system as well as potentially parvocellular neurons that project to the median eminence (Silverman et al., 1990). Following an IP injection of 600 µg of FG, all SON OT neurons were FG-immunoreactive. Most (∼96%) of the OT neurons in the anterior and middle extent of PVN were also FG positive (Figure 6 A–K). However, a cluster of non-FG labeled OT neurons was observed in the dorsal part of the posterior PVN (Figure 6C,F,I,L), and isolated non-FG neurons were scattered throughout the extent of PVN.
Figure 6.
Organization of the neurohypophysial system of the paraventricular nucleus of the hypothalamus (PVN). Prairie voles were injected intraperitoneally with FG and brain sections were processed for FG and OT immunoreactivity. OT immunoreactivity in the anterior (A) middle (B) and posterior (C) regions of the PVN as revealed with the immunoperoxidase method. D–F) Micrographs of immunofluorescent (Texas Red-labeled) OT-containing neurons in sections of the hypothalamus representative of those shown in A–C. G–I) FG-labeled cells labeled with Alexa Fluor 350 in the same sections shown as in D-F. J-I) Overlay of OT and FG labeling in D-I. Notice that the majority of cells in the anterior and middle levels of the PVN are labeled for both OT and FG, suggesting that they are magnocellular neurohypophysial neurons. Notice the two populations of cells in F, a small dorsal and a larger ventral group. The dorsal cluster does not have FG immunoreactivity suggesting that these are the parvocellular OT cells that project to the hindbrain and spinal cord. Scale bar in C = 200 µm (valid for A–B); bar in L = 100 µm (valid for D–K). 3V = third ventricle.
DISCUSSION
OT modulates a wide range of social behaviors, including maternal nurturing and bonding, sexual behavior, and social attachment. In humans, intranasal OT enhances interpersonal trust, eye gaze, recognition of familiar faces, and the ability to infer the emotions of others (Kosfeld et al., 2005, Domes et al., 2007, Guastella et al., 2008, Savaskan et al., 2008). Yet remarkably little is known about the origins of neurons that modulate these behaviors. In prairie voles, pharmacological and genetic manipulation studies have suggested that OTRs in the NAcc are involved in regulating spontaneous maternal behavior and partner preference formation (Young et al., 2001, Olazabal and Young, 2006a, Ross et al.). Here we provide the first demonstration that extracellular OT concentrations in the NAcc increase during sociosexual interactions with a male. In this study, despite using the highest sensitivity of radioimmunoassay, basal levels of OT were undetectable due to the low recovery rate of OT through the microdialysis tubing (∼2%) (Neumann et al., 1993) and the low density of OT fibers within the NAcc Therefore, it is important to note that we cannot conclude that the NAcc is devoid of extracellular OT under basal conditions or following exposure to a male without mating. Rather, the concentrations were simply below the level of detection. However, the fact that 38.5% of the females that mated produced microdialysates with measurable OT provides evidence that OT is released from the OT-immunoreactive processes coursing through the NAcc during free interactions with a male that includes mating We also cannot conclude that mating is the stimulus that triggers OT release from our studies, although vaginocervical stimulation has been shown to result in increased central OT release in rats (Sansone et al., 2002). Indeed 2 of the 13 females that did not receive an intromission because they were not sexually receptive produced microdialysis samples with measurable OT, but this failed to be statistically different from baseline. It is plausible that social interaction, including mounting attempts, also results in OT release, albeit to a lesser extent than what occurs with mating. This would be consistent with the observation that female prairie voles can form partner preferences following cohabitation with a male without mating, although this requires longer time periods (Williams et al., 1992). Indeed, OT release within the PVN has been reported in male rats during restricted exposure to a female (Waldherr and Neumann, 2007). However, it is important to note that when we separately analyzed the OT samples from the females that mated during the free exposure and those that did not mate, only the group of females that mated produced a statistically significant increase in the number of animals with detectable OT compared to the restricted exposure.
An increase in central extracellular OT following mating is consistent with studies in sheep and rats, that demonstrate that vaginocervical stimulation results in central OT release (Kendrick et al., 1986, Sansone et al., 2002). Vaginocervical stimulation occurs naturally during parturition and mating. The OT released during parturition likely plays a role in the onset of maternal behavior (Pedersen and Prange, 1979, Kendrick et al., 1991), while OT released during mating, at least in prairie voles, facilitates the formation of a pair bond. A recent study also reported that OT is released in the hypothalamus of male rats following mating (Waldherr and Neumann, 2007). The role of OT in facilitating partner preference in male prairie voles is not clear, as one study found that OT or OT antagonist administered ICV did not effect partner preference formation (Winslow et al., 1993); while another lab found central OT infusion in males did facilitate partner preference formation (Cho et al., 1999). In humans, OT has been reported to increase in the plasma during sexual arousal and ejaculation or orgasm, although changes in central OT concentrations have not been examined (Carmichael et al., 1987).
The low levels of OT in the microdialysate raises the question of whether the concentration of OT in the extracellular space is physiologically significant. Assuming a 2% recovery rate for OT in the microdialysate, samples with OT content of 0.05 pg/25 µl dialysate would be recovered if the extracellular concentration of OT was 100 pg/ml, or ∼0.1 nM, which is near the IC50 of OT for the OTR of 0.28 nM (Kimura et al., 1997). Thus it is likely that OT concentrations in the NAcc reach physiologically significant levels during sociosexual interactions in female prairie voles.Our results also illustrate the remarkable evolutionary conservation of OT innervation in the ventral striatum despite marked species differences in receptor distribution. Prairie voles have high densities of OTR in the NAcc, while meadow voles and mice are virtually devoid of receptors in this region (Insel and Shapiro, 1992, Olazabal and Young, 2006b). We expected to find similar species differences in OT fibers in the NAcc. However, each species displayed a semiquantitatively similar diffuse pattern of OT-immunoreactive fibers in the NAcc. A recent report described similar OT-immunoreactive fibers in the NAcc of the eusocial naked-mole rat (Rosen et al., 2008). The contrast between the conservation of distribution of peptide and receptor suggests that diversity in receptor distribution, and not OT projections, drives the evolution of central OT function. As can be seen in the horizontal slice, OT fibers penetrate diffusely through the forebrain rather than traveling in discrete tracts targeting specific regions, potentially providing a source of OT to diffuse forebrain areas with OT receptors. This is consistent with the observation that OT released from fibers can diffuse to target regions, in a paracrine fashion (Ludwig and Leng, 2006). It should be noted, however, that we did not control for the estrous cycle in the rats or mice, and variation in OT content in these fibers could occur over the estrous cycle.
The electron microscopic analysis revealed that the majority of OT-immunoreactive elements in the NAcc are large diameter (∼200nm), unmyelinated processes packed with dense core vesicles. OT-immunoreactive elements containing asymmetric synapses were also clearly present. Interestingly, these synapses were largely devoid of OT-containing dense core vesicles, but instead contained smaller synaptic vesicles. Hypothalamic OT neurons exhibit somatic and dendritic release of dense core vesicles (Pow and Morris, 1989, Landgraf and Neumann, 2004, Ludwig and Leng, 2006). Based on these ultrastructural observations, we hypothesize that OT may be released from these en passant processes in a mechanism similar to somato-dendritic release in the hypothalamus (Pow and Morris, 1989). Magnocellular OT neurons express vesicular glutamate transporter, suggesting that they are glutamatergic (Ponzio et al., 2006), and have unmyelinated axons (Swanson and Sawchenko, 1983). Likewise, the asymmetric specialization of the synapses seen on OT-immunoreactive terminals in the NAcc of prairie voles is indicative of glutamatergic neurotransmission. It should be noted that we could not definitively demonstrate that the OT-containing elements were axons or extended dendrites. However, the presence of synapses associated with some terminals suggests that at least some fibers are axonal projections.
The results from the retrograde tract tracing studies are most consistent with the hypothesis that NAcc OT fibers originate from the PVN and SON. This result was surprising since neither of these regions have been reported as being labeled following injection of retrograde tracers into the rat NAcc (Phillipson and Griffiths, 1985, Brog et al., 1993). Initially we hypothesized that FG leaking into the capillary system or into the ventricles may have been producing false positive FG-labeled cells. However, our control infusions of similar amounts of FG directly into the lateral ventricle or IP did not result in detectable FG labeling in the PVN or SON. The double-labeled neurons that were identified were strongly labeled while surrounding PVN and SON neurons were not labeled, which also argues against leakage of the FG into the bloodstream. We suspect that the low density of the OT projections in the NAcc and the sparseness of OT-containing axon terminals, the main sites of FG uptake (Ju et al., 1989), limited considerably the amount of tracer being taken up by the OT projection system. Consequently, only a few retrogradely labeled PVN or SON neurons were detected in 6 out of 10 animals with FG injections into the NAcc. This phenomenon has also been seen on other neuronal systems which led to controversy regarding the degree of axonal collateralization of the striatofugal system to both segments of the globus pallidus (see (Parent et al., 2000) for review). We suspect that the sparseness of OT-containing axon terminals actually resulted in an underrepresentation of the OT neurons that project to the NAcc as determined by FG uptake. However, although the number of double-labeled OT neurons was small, they were only seen in the hypothalamic brain areas of the PVN and SON. This finding is consistent with lesion studies that found a loss of OT-immunoreactive fibers after PVN or SON ablation (De Vries and Buijs, 1983, Hawthorn et al., 1985). In addition, it has been estimated that release of a single dense core vesicle contains enough OT to activate it OT receptors in the local vicinity (Leng and Ludwig, 2008). In summary, while the number of OT neurons that were labeled with FG was low, this may be an under estimation. Furthermore, given the high concentrations of OT in each dense core vesicle, the density of OT fibers in the NAcc is likely sufficient to release physiologically significant amounts of OT into this region. affect social behavior.
Early retrograde tracing studies examining the OT projections of the hindbrain and brainstem indicated that those projections arise from parvocellular OT neurons in the PVN (Ono et al., 1978, Swanson and Sawchenko, 1983). This has led to the extrapolation by many in the field that forebrain OT projections arise from the parvocellular PVN neurons, while the magnocellular neurons of the PVN and SON project exclusively to the posterior pituitary. Our results bring that assumption into question. First, all SON neurons are magnocellular (Swanson and Sawchenko, 1983), yet a few SON neurons were clearly FG-positive following infusion into the NAcc. In addition, within the PVN of prairie voles, non-pituitary projecting OT neurons were clustered in the dorsal posterior region. These neurons may be giving rise to the hindbrain and brainstem OT system. However, FG-positive OT neurons were not found in this population, but were most often found in the anterior PVN close to the third ventricle, an area predominated by neurohyophyseal neurons. In prairie voles, OT soma are also found in the medial preoptic area, median preoptic nucleus, bed nucleus of the stria terminalis, and lateral hypothalamic area (Wang et al., 1996). However, we did not detect any FG-labeled OT neurons in these regions. The large caliber, and unmyelinated nature of the NAcc OT fibers is also consistent with magnocellular origin (Harris et al., 1969), however it should be noted that parvocellular neuronal axons are often also unmyelinated (van Leeuwen et al., 1978). In the horizontal slices, the OT fibers coursing toward the NAcc appear to emerge from the axonal projections of the neurohypophyseal system. Given the low detection rate of double-labeled OT neurons, future studies using alternative techniques, are needed before definitive conclusions are drawn regarding the origin of the OT fibers in the NAcc.
While not examined in the present study, we hypothesize that the NAcc OT fibers may be from collaterals of the magnocellular neurohypophysial OT neurons. In the mouse, a minority of PVN magnocellular neurons produce collaterals around the level of the fornix that turn anteriorly and become perpendicular to the section, exactly as would be expected if going to the NAcc (Hatton et al., 1985). If the prairie accumbal OT fibers are indeed collaterals of magnocellular hypothalamic neurons, this would provide a direct mechanism for coordination of central release deep in the forebrain with peripheral release under the appropriate physiological conditions, such as vaginocervical stimulation during mating or parturition, or sensory stimulation during suckling. Indeed, it is possible that extracelluar OT in the NAcc reflects a combination of somatodendritic release within both the PVN and SON that diffuses to the NAcc, and en passant or terminal release from OT fibers located in the NAcc and originating from PVN and SON. These multiple modes of central release of OT may explain high temporal and spatial resolution and a theoretically unlimited variability in OT signaling modes (Landgraf and Neumann, 2004). While central and peripheral release patterns may be coordinated to trigger synergistic effects, local release independent of peripheral secretion may also be possible (Neumann et al., 1993, Landgraf and Neumann, 2004, Ludwig and Leng, 2006).
The present study provides the most complete characterization of the OT system involved in regulating affiliative behavior to date. We demonstrate that extracellular OT concentrations in the NAcc increase with sociosexual interactions with a male in the female prairie vole. OTR antagonist infused in this region block mating-induced partner preference formation as well as alloparental behavior (Young et al., 2001, Olazabal and Young, 2006a). This OT projection pattern is not an oddity of the monogamous prairie vole, but represents an evolutionary conserved forebrain projection. The ultrastructure and the diffuse nature of the projections are consistent with a paracrine, neuromodulatory function of forebrain OT. Finally, if these projections are indeed collaterals of magnocellular OT neurons of the neurohypophysial system, they provide a mechanism of coordination of peripheral physiology and behavior necessary for reproductive success.
Acknowledgements
The authors want to thank Hemu Nair for his assistance in statistical analysis, Tig Rainnie for his gift of antibody and technical assistance, and Lorra Mathews for her excellent job managing our vole colony. Thanks are also due to Jean-Francois Pare and Susan Jenkins for their help with the electron microscopy immunocytochemistry procedures and data collection presented in this manuscript. This study was supported by NIH grants MH064692 to LJY, RR00165 to Yerkes National Primates Research Center, NSF STC IBN-9876754 and a collaborative DAAD / NSF grant (IDN, LJY).
Abbreviations
- DAB
3,3′-diaminobenzidine
- BT
biotin-tyramine
- EM
electron microscopy
- FG
Fluorogold
- FM
fluorescent microscopy
- IP
intraperitoneally
- LM
light microscopy
- NAcc
nucleus accumbens
- OT
oxytocin
- OTR
oxytocin receptors
- PVN
paraventricular
- PB
potassium buffer
- KPBS
potassium phosphate-buffered saline
- SON
supraoptic nuclei
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bargmann W. Uber die neurosekretorische Verknupfung von Hypothalamus und Neurohypophyse. Z Zellforsch Mikrosk Anat. 1949;34:610–634. [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Young LJ, Russell J. Oxytocin: synthesis, secretion, and reproductive functions. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. New York: Elsevier; 2006. pp. 3055–3127. [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Harris GW, Manabe Y, Ruf KB. A study of the parameters of electrical stimulation of unmyelinated fibres in the pituitary stalk. J Physiol. 1969;203:67–81. doi: 10.1113/jphysiol.1969.sp008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Cobbett P, Salm AK. Extranuclear axon collaterals of paraventricular neurons in the rat hypothalamus: intracellular staining, immunocytochemistry and electrophysiology. Brain Res Bull. 1985;14:123–132. doi: 10.1016/0361-9230(85)90072-3. [DOI] [PubMed] [Google Scholar]
- Hawthorn J, Ang VTY, Jenkins JS. Effects of Lesions in the Hypothalamic Paraventricular, Supraoptic and Suprachiasmatic Nuclei on Vasopressin and Oxytocin in Rat-Brain and Spinal-Cord. Brain Research. 1985;346:51–57. doi: 10.1016/0006-8993(85)91093-5. [DOI] [PubMed] [Google Scholar]
- Horvath TL. An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. J Neurosci. 1998;18:1546–1558. doi: 10.1523/JNEUROSCI.18-04-01546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ju G, Han ZS, Fan LZ. Fluorogold as a retrograde tracer used in combination with immunohistochemistry. J Neurosci Methods. 1989;29:69–72. doi: 10.1016/0165-0270(89)90109-x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. Cerebrospinal fluid levels of acetylcholinesterase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep. Neuroendocrinology. 1986;44:149–156. doi: 10.1159/000124638. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep. Brain Res Bull. 1991;26:803–807. doi: 10.1016/0361-9230(91)90178-m. [DOI] [PubMed] [Google Scholar]
- Kimura T, Makino Y, Bathgate R, Ivell R, Nobunaga T, Kubota Y, Kumazawa I, Saji F, Murata Y, Nishihara T, Hashimoto M, Kinoshita M. The role of N-terminal glycosylation in the human oxytocin receptor. Mol Hum Reprod. 1997;3:957–963. doi: 10.1093/molehr/3.11.957. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Neurons with access to the general circulation in the central nervous system of the rat: a retrograde tracing study with fluoro-gold. Neuroscience. 1991;44:655–662. doi: 10.1016/0306-4522(91)90085-3. [DOI] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishino H, Sasaka K. Paraventricular Nucleus Connections to Spinal-Cord and Pituitary. Neurosci Lett. 1978;10:141–146. doi: 10.1016/0304-3940(78)90025-3. [DOI] [PubMed] [Google Scholar]
- Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 2000;23:S20–S27. doi: 10.1016/s1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Ponzio TA, Ni Y, Montana V, Parpura V, Hatton GI. Vesicular glutamate transporter expression in supraoptic neurones suggests a glutamatergic phenotype. J Neuroendocrinol. 2006;18:253–265. doi: 10.1111/j.1365-2826.2006.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–817. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in Oxytocin Receptor Density in the Nucleus Accumbens has Differential Effects on Affiliative Behaviors in Monogamous and Polygamous Voles. tJ Neurosci. In Press doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the Neural Mechanisms Regulating Social Cognition and Affiliative Behavior Front Neuroendocrinol. In Press doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone GR, Gerdes CA, Steinman JL, Winslow JT, Ottenweller JE, Komisaruk BR, Insel TR. Vaginocervical stimulation releases oxytocin within the spinal cord in rats. Neuroendocrinology. 2002;75:306–315. doi: 10.1159/000057340. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Roth KA. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem. 1996;44:1331–1335. doi: 10.1177/44.11.8918908. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Witkin JW, Silverman RC, Gibson MJ. Modulation of gonadotropin-releasing hormone neuronal activity as evidenced by uptake of fluorogold from the vasculature. Synapse. 1990;6:154–160. doi: 10.1002/syn.890060206. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, Swaab DF, de Raay C. Immunoelectronmicroscopic localization of vasopressin in the rat suprachiasmatic nucleus. Cell Tissue Res. 1978;193:1–10. doi: 10.1007/BF00221596. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]