Abstract
The hematopoietic and vascular lineages are intimately entwined as they arise together from bipotent hemangioblasts and hemogenic endothelial precursors during human embryonic development. In vitro differentiation of human pluripotent stem cells toward these lineages provides opportunities for elucidating the mechanisms of hematopoietic genesis. We previously demonstrated the stepwise in vitro differentiation of human embryonic stem cells (hESC) to definitive erythromyelopoiesis through clonogenic bipotent primitive hemangioblasts. This system recapitulates an orderly hematopoiesis similar to human yolk sac development via the generation of mesodermal-hematoendothelial progenitor cells that give rise to endothelium followed by embryonic primitive and definitive hematopoietic cells. Here, we report that under modified feeder-free endothelial culture conditions, multipotent CD34+CD45+ hematopoietic progenitors arise in mass quantities from differentiated hESC and human induced pluripotent stem cells (hiPSC). These hematopoietic progenitors arose directly from adherent endothelial/stromal cell layers in a manner resembling in vivo hematopoiesis from embryonic hemogenic endothelium. Although fibroblast-derived hiPSC lines were previously found inefficient in hemato-endothelial differentiation capacity, our culture system also supported robust hiPSC hemato-vascular differentiation at levels comparable to hESC. We present comparative differentiation results for simultaneously generating hematopoietic and vascular progenitors from both hESC and fibroblast-hiPSC. This defined, optimized, and low-density differentiation system will be ideal for direct single-cell time course studies of the earliest hematopoietic events using time-lapse videography, or bulk kinetics using flow cytometry analyses on emerging hematopoietic progenitors.
Key terms: human embryonic stem cells (hESC), induced pluripotent stem cells (iPSC), hematopoietic stem-progenitor cells, vascular progenitor cells, hemogenic endothelium, hemovascular differentiation, flow cytometry
The recent advent of cell reprogramming and induced pluripotency (1, 2) has opened new avenues of investigation for disease modeling, drug discovery (3–5), and patient-specific cell therapies (6). In vitro differentiation models for dissecting the developmental processes that give rise to complex cell lineages such as the hematopoietic system have now become possible (7). The hematopoietic system develops by means of various transitory cell lineages throughout fetal life. Two blood-forming sites have been identified during human embryonic development: the extraembryonic yolk sac (YS) and the aorta-gonad-mesonephros (AGM), both of which exhibit a close juxtaposition of hematopoietic and vascular lineages (8). The existence of a common ephemeral precursor for embryonic hematopoiesis and vasculogenesis termed the hemangioblast has been supported by work on human embryonic stem cells (hESC) (9, 10). However, in the adult, intraembryonic hematopoiesis originates in the dorsal aorta (DA) from a pool of specialized endothelial cells termed hemogenic endothelium (11–14). In an effort to understand the delicate equilibrium between adult versus embryonic hematopoietic and endothelial fate through ontogeny, we and others have developed methodologies based on the differentiation of pluripotent stem cells that generate YS-like clonogenic bipotent precursors (9) or DA-like intermediate mesodermal progenitors (7, 15–17).
The derivation of engraftable vascular and hematopoietic stem cells (HSC) from patient-specific human induced pluripotent stem cell (hiPSC)-derived hemangioblasts or hemogenic endothelium may have great clinical utility for the effective, long-term treatment of hemato-vascular disorders. However, recent studies have suggested that hiPSC do not produce hemato-endothelial progeny in a manner that is quantitatively and qualitatively comparable to hESC (18). There may be several etiologies for this limitation, including the quality of reprogramming achieved in fibroblast-iPSC (due to retention of somatic donor epigenetic memory), the method of hiPSC culture used for maintaining pluripotency (e.g. on murine embryonic fibroblasts (MEF) vs. feeder-free monolayer), and the inherent efficiency of the differentiation protocol (e.g., embryoid body vs. stromal co-culture-based).
In these studies, we focused on optimizing our previously described human embryoid body (hEB)-based hemato-endothelial differentiation method for efficient hiPSC differentiation (10). We report that with rigorous culture technique for pluripotency maintenance, and optimized endothelial-supporting culture conditions, the relatively diminished hemato-endothelial differentiation capacities of fibroblast-hiPSC (18–20) can be considerably improved to levels comparable to hESC. We describe a defined differentiation system which is adapted from our previously described hEB differentiation method, and utilizes a minimal combination of recombinant growth factors [bone morphogenetic protein-4 (BMP4), vascular endothelial growth factor (VEGF), and fibroblast growth factor-2 (FGF2)], followed by adherent low-density culture in well-defined endothelial growth medium (EGM-2). In this accessible two-dimensional (2D) culture system, multipotent hematopoietic CD34+CD45+ progenitors arose directly in mass quantities and bud off from adherent hemogenic endothelial cells. This efficient differentiation system can be used for direct time-lapse videography studies, time-course studies of hematopoietic genesis events (e.g., from various hiPSC disease models), or kinetic flow cytometry analyses of newly emerging, floating CD34+CD45+ hematopoietic progenitors.
Materials and Methods
Cell Culture and Differentiation Protocols
hESC and hiPSC cultures
hESC [H9 (WA09; WiCells, Madison, WI)] and lentivirally-generated fibroblast hiPSC (IMR90-1 and IMR90-4,WiCells) (2) lines were maintained in undifferentiated states and expanded as previously described on irradiated (5,000 cGy) mouse embryonic fibroblasts (MEF) (10, 21). The study was approved by the Embryonic Stem Cell Research Oversight Committee at the Johns Hopkins School of Medicine (application No. ESC09-06-25-01) MEF were derived in our laboratory using CF1 (Charles River, Wilmington, MA) and DR4 (The Jackson Laboratory, Bar Harbor, ME) mouse embryos at E13.5. hESC and hiPSC cultures were maintained exclusively on MEF at their second and third passage (see Supporting Information Table S1 for detailed MEF medium composition). hESC/hiPSC were grown in Dulbecco’s modified eagle medium (DMEM)/F12 supplemented with 20% knockout serum replacement, 0.1 mM minimum essential medium (MEM)-non-essential amino acid, 1 mM l-glutamine, 0.1 mM beta-mercaptoethanol, and 4 ng/mL FGF2 (all Life Technologies, Grand Island, NY; Supporting Information Table S1). Spontaneously differentiating colonies displaying aberrant morphology were manually discarded by micropipette-ablation using an inverted microscope (Eclipse-TS100, Nikon Instruments Inc., Melville, NY) in a laminar flow cabinet (MidAtlantic Diagnostics Inc., Mount Laurel, NJ). Cells were passaged enzymatically using a solution of collagenase type-IV (1 mg/mL in DMEM/F12, 5 min, 37°C, Sigma, St. Louis, MO, Cat No. 17104-019) or manually dissected using a 10 µL micropipette (Eppendorf, Hamburg, Germany) every 6–7 days when they reached 70–80% confluency. hESC and hiPSC were routinely checked for pluripotency marker expression [OCT-4, Nanog, stage specific embryonic antigen (SSEA4), Tra1-61, Tra1-80, see Supporting Information Fig. S1] by flow cytometry.
Vascular and Hematopoietic Differentiation
hEB generation
Vascular differentiation conditions were adapted from our previously described hEB protocol (9, 10). hESC and fibroblast-hiPSC lines with >95% undifferentiated morphology were expanded in six-well plates until 80–90% confluency. Culture medium was switched to adaptation medium (AM, see Supporting Information Table S2 for detailed medium composition) for 1 day prior to hEB generation (Table 1). On the next day, pluripotent colonies were treated with 1 mL of 2 mg/mL dispase (Sigma, Cat No. 17105-041) in DMEM/F12 for 5 min at 37°C. The dispase solution was removed, and cell clumps were collected by cell scraping (cell scraper, Sarstedt, Newton, NC) with 1 mL of DMEM/F12. Cells were washed twice in DMEM/F12, and disaggregated by gentle pipetting. Cell suspensions were centrifuged (200 g, 5 min, room temperature), and cell pellets were resuspended in AM (at a concentration of 1 to 2 × 106 cells/mL). ESC/hiPSC suspensions of 0.5 mL were gently deposited over 2 mL of semi-solid methylcellulose medium (MM, see Supporting Information Table S2) in ultra-low attachment six-well plates (Corning, Lowell, MA, Cat No. 3471) using a 5 mL syringe (BD, Franklin Lakes, NJ, Cat No. 309603) and a 16 gauge needle (BD, Cat No. 305198) followed by gentle homogenization. Plates were tightly wrapped using Saran Wrap (SC Johnson, Racine, WI) to create semi-hypoxic conditions, and incubated at 37°C. Two days later, each three wells of small aggregated hEB were collected in 40 mL of phosphate buffer saline (PBS, Life Technologies), centrifuged at 300 g for 5 min at room temperature, washed once more in PBS, and re-suspended in liquid differentiation medium (LDM, see Supporting Information Table S2). The hEB/LDM suspension was returned to the original ultra-low attachment plate and reincubated in hypoxic conditions. hEB were collected every 2 days and resuspended in fresh LDM with supplement of growth factors. A detailed description of this protocol is also available in Ref. 15.
Table 1.
Stepwise procedure for the optimized hemato-vascular differentiation of human pluripotent stem cells
| DAYS OF DIFFERENTIATION | NAME OF MEDIUM | PURPOSE | CULTURE DIMENSION |
|---|---|---|---|
| Day 0 | hESC mediuma | Undifferentiated state | 2D |
| Day 1 | Adaptation medium (AM)a | Pre-differentiation | 2D |
| Day 1 to 3 | Methylcellulose medium (MM)a | Forming hEBs, mesodermal lineage commitment | 3D |
| Day 3 to 8 | Liquid differentiation medium (LDM)a | Differentiate into mesodermal lineage progenitor cells | 3D |
| Day 8 to 15 | EGM-21VEGF (25ng/ml) | Stromal vascular and hematopoietic differentiation | 2D |
| Non-adherent cellsb | Methylcellulose hematopoietic colony assay medium | Enumerate hematopoietic progenitor cells | 3D |
| Post sortb | EGM-2 | Purify vascular progenitor fractions | 2D |
| Time frame | 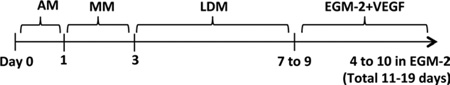 |
||
For detailed medium composition, see Supplementary Tables 1 and 2
Cells are derived from day 8 EB with additional differentiation in EGM-2 culture
EGM-2 hemogenic endothelial culture
The expression of cell surface markers for hematopoietic and endothelial cells (CD31, CD34, and CD143 (angiotensin-converting enzyme, ACE, aka BB9) were evaluated by flow cytometry during hEB differentiation, and the 8th day of 3D culture was determined as the optimal time point to harvest putative mesodermal endothelial progenitors. Accordingly, hEB which had been cultured in LDM for 5 days (Table 1) were washed in PBS, enzymatically digested with collagenase type-IV (1 mg/mL in DMEM/F12) for 5 min at 37°C, and disaggregated using a 1 mL pipette (Gilson, Middleton, WI). hEB cells were collected in MEF medium (see Supporting Information Table S1 for composition) to neutralize collagenase activity and centrifuged (200g, 5 min, ambient temperature). Cells were counted using the automated cell counter Countess (Life Technologies), plated into fibronectin (10 µg/mL in PBS, overnight at 4°C, Life Technologies, Cat No. 33016-015) pre-coated six-well plates (Greiner Bio-One, Monroe, NC, Cat No. 657960) at a density of 1–1.5 × 105 hEB cells/well, and cultured in complete Endothelial Growth Medium™-2 (EGM-2, Lonza, Walkersville, MD, Cat No. CC-3162) BulletKit medium, supplemented with 25 ng/mL VEGF (Life Technologies). Fresh VEGF-supplemented EGM-2 was replenished the following day and every 2 days thereafter. Large amounts of non-adherent cells emerging from these monolayer cultures were collected daily and further analyzed by flow cytometry or tested for their hematopoietic potential using methylcellulose colony forming unit (CFU) assay.
Hematopoietic Methylcellulose CFU Assays
hEB were enzymatically treated to obtain single cell suspensions, as described below (see section “hESC/hiPSC, hEB, and adherent and non-adherent EGM-2 culture-derived hEB cells”), for determination of hematopoietic progenitor frequency (9, 10). Alternatively, emerging floating cells were harvested from VEGF-supplemented EGM-2 cultures at various time points and assayed for hematopoietic potential by methylcellulose CFU assays as previously described (10, 15). Briefly, either 3 × 105 single hEB cells or floating cells were resuspended in 450 µL of StemSpan® SFEM medium (serum-free expansion medium, StemCell™ Technologies, Vancouver, BC, Canada) and deposited into 4.05 mL of MethoCult® serum free methylcellulose medium (MethoCult® SF H4436, StemCell™ Technologies, Cat No. 04436). The 4.5 mL of cell/methylcellulose mixture were gently vortexed and let to sit down for 5 min to avoid air bubbles prior to load into three 35 mm grid dishes (1.5 mL/dish, Nalge Nunc International, Rochester, NY, Cat No. 174926). Hematopoietic colony formation was assayed between 14 to 21 days of culture. Hematopoietic colonies were counted and photographed using an inverted microscope (Eclipse Ti-u, Nikon Instruments Inc.), and harvested for flow cytometry analysis. If blast immature colonies were still predominant after 10 to 14 days of culture in methylcellulose, 0.5 mL of fresh MethoCult culture medium were added to the culture, and the cells were allowed to grow until 3 weeks of differentiation. Hematopoietic CFU efficiency of EGM-2 culture-derived floating cells was compared to hEB cells (Fig. 4B). hEB cells were maintained in LDM medium for matching timepoints of EGM-2 subculture conditions before disaggregation and methylcellulose CFU assay.
Figure 4. Hematopoietic cells generated from EGM-2 culture condition.
(A) Floating, non-adherent cells were collected from EGM-2 hEB cultures. Day 8 hEB were cultured as described in the text for an additional 3, 5, and 8 days in EGM-2 culture, and analyzed by flow cytometry for surface expression of CD34, CD143/ACE, and CD45. (B) Hematopoietic CFU of floating, non-adherent cells generated in EGM-2 cultures from differentiated H9 cells at different time points (left panel). Floating cells from day 8 EB with additional day 3 in EGM-2 culture (total day 11) of H9, IMR90-1 (IMR-1), and IMR90-4 (IMR-4) were compared for hematopoietic CFU assay (right panel). (C) Hematopoietic colonies from methylcellulose cultures were pooled and analyzed by flow cytometry for expression of glycophorin A (CD235a) and embryonic (Hb-ε), fetal (Hb-F), and adult (Hemoglobin β chain, HbA) hemoglobin expression. Inserts are appropriate isotype controls. Shown are representative experiments performed three times.
Flow Cytometry
Validation of cellular viability
Flow cytometry samples were pre-stained with the LIVE/DEAD® Fixable Red Dead Cell Stain Kit for 488-nm excitation (Invitrogen, Cat No. L-23102) as per manufacturer instructions. Single cell suspensions were prepared as described below. Cells were resuspended in PBS at a concentration of 1 × 106 cells/mL. Cells were stained by addition of 1 µL of DMSO-reconstituted dye per microliter of cell suspension and incubated for 30 min at 4°C. Samples were washed in PBS and fixed in 1% formaldehyde, before acquisition on a FACSCalibur™ flow cytometer (Supporting Information Fig. S2).
hESC/hiPSC, hEB, and adherent and non-adherent EGM-2 culture-derived hEB cells
hEB and both the adherent and non-adherent cellular fractions of EGM-2 cultures were collected at multiple time points. hEB were washed twice in PBS, and enzymatically digested with Accutase® (Life Technologies, 5 min, 37°C). Gentle hEB dissociation was completed by three to four passages through a 21 gauge needle and a second 5 min incubation at 37°C. Enzymatic activity was neutralized using MEF medium, and the resulting cell suspension was filtered through a 40 µm cell-strainer (Fisher Scientific, Pittsburgh, PA, Cat No. 22363547) to eliminate cell clusters. Cells were centrifuged (200g, 5 min, ambient temperature) and resuspended in staining buffer (phenol red free EGM-2:PBS, 1:1) at a concentration of 1 × 106 cells/mL. Single cell suspensions (1 × 105 cells in 100 µL per tube) were incubated for 20 min on ice with directly conjugated mouse monoclonal anti-human antibodies (2 µL of CD31-APC (eBioscience, San Diego, CA, Cat No. 17-0319-73, Clone WM59), or 5 µL each of CD34-PE (BD Biosciences, San Jose, CA, Cat No. 555822, Clone 581), CD45-APC (BD Biosciences, Cat No. 555485), CD143-APC (BD Biosciences, Cat No. 557929, Clone HI30), CD146-PE (BD Biosciences, Cat No. 550315, Clone P1H12), CD133-PE (Miltenyi-Biotec, Cat No 130-080-801, Clone AC133), and KDR-APC (R&D System, Cat No. FAB357A, Clone 89106) in solo or duo combination. Isotype controls matching each immunoglobulin subtypes were stained analogously to control for nonspecific binding. Cells were washed with 3 mL of PBS, centrifuged (300g, 5 min, room temperature), and resuspended in 300 µL of staining buffer prior to acquisition. Viable cells were analyzed readily using a duallaser FACSCalibur flow cytometer (BD Biosciences) equipped with blue argon (488 nm) and red diode (635 nm) lasers. At least 10,000 events were acquired for each tube using the BD CellQuest Pro analytical software (BD Biosciences), maintaining an acquisition rate below 300 event/s. Autofluorescence was corrected using unstained controls and live gating was approximated using light scatter properties (linear SSC × linear FSC), as pre-staining cell counts using trypan blue and hemocytometer or automated cell counter Countess™ were consistently indicative of a cellular viability over 90–95%. All data files were subsequently analyzed offline using Flowjo analysis software (Tree Star, Asland, OR).
Undifferentiated hESC/iPSC
Seventy to 80% confluent hESC and hiPSC colonies were washed once in PBS and treated in 0.05% trypsin-ethylene-diamine-tetraacetic acid (EDTA) (Life Technologies) or Accutase for 5 min at 37°C. After neutralization of enzymatic activity with hESC medium, single cell suspensions were filtered through a 40 µm cell-strainer and centrifuged (200g, 5 min, room temperature). Cell pellets were resuspended in 10% hESC medium in PBS at a concentration of 1 × 106 cells/mL. 100 µL of this cell solution were stained by addition of monoclonal mouse antihuman antibodies SSEA4-APC (2 µL, R&D Systems, Minneapolis, MN, Cat. No. FAB1435A, Clone MC-813-70), Tra-1-60-PE (5 µL, BD Biosciences, Cat No. 560193, Clone TRA-1-60), Tra-1-81-PE (5 µL, BD Biosciences, Cat No. 560161, Clone TRA-1-81), OCT-4-PE (20 µL, R&D Systems, Cat No. IC1759P, Clone 240408) antibodies, or unconjugated polyclonal goat anti-human Nanog (20 µL, R&D Systems, Cat No. AF1997) antibody followed by an incubation with secondary goat anti-mouse-APC (1:500, BD Biosciences, Cat No. 550826) antibody after one wash with 3 mL of PBS and centrifugation (300 g, 5 min ambient temperature). For staining of OCT-4 and Nanog, cells were formerly fixed and permeabilized using Fix & Perm Cell Fixation and Permeabilization kit (Life Technologies, Cat No. GAS-003) prior to adding antibodies. All antibody incubations were performed for 20 min on ice. Cell acquisition and data analysis were executed as described above.
Hematopoietic colonies
Hematopoietic colonies were enumerated under a phase contrast microscope (Eclipse Ti-u). Cells were collected after addition of PBS, 14 to 21 days post-MethoCult culture and washed thoroughly in PBS to remove any residual methylcellulose medium. Cell solutions were filtered through a 40 µm cell strainer, and centrifuged at 200g for 5 min (room temperature). Cell pellets were resuspended in 5% FBS/PBS solution and stained (20 min on ice) for cell surface antigens with mouse monoclonal anti-CD235a (glycophorin A)-PE (1 µL BD Biosciences, Cat No. 555570) antibody. For intracytoplasmic hemoglobin staining, cells were fixed for 20 min in 100 µL Reagent A from the Fix & Perm Cell Fixation and Permeabilization kit (Life Technologies). Following fixation, cells were washed in 3 mL PBS, resuspended in 100 µL Reagent B and stained for 20 min (on ice) with 0.5 µL of mouse anti-human hemoglobin-ε-FITC (Fitzgerald Industries International, Cat No. 61C-CR8008M1F, Clone 90050), 1 µL of mouse anti-human hemoglobin-F-FITC (BD Biosciences, Cat No. 552829, Clone 2D12), or 2 µL of unconjugated mouse anti-human hemoglobin-β antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, Cat No. sc-21757, Clone 37-8). For hemoglobin-β antibody staining, goat anti-mouse secondary IgG1-PE antibody (1:500, Southern Biotech, Birmingham, AL, Cat. No. 1707-09) was added after washing and incubated for 20 min on ice. Cells were finally washed in PBS and resuspended in 100 µL of 5% FBS/PBS solution prior to acquisition. Cell acquisition and data analysis were performed as described above. Spectral compensation was achieved using single color stained controls and BD CellQuest Pro analytical software.
Purification of hEB-Derived Angioblast Populations Via Surface Expression of CD31 and CD146
Purification of EGM-2 culture-derived vasculogenic cell populations by cell sorting
Day 8 hEB were plated onto fibronectin coated plates in EGM-2 medium supplemented with 25 ng/mL VEGF, as described above. After 4 to 6 days of culture into VEGF-supplemented EGM-2, adherent cells were washed in PBS, treated with 0.05% trypsin-EDTA (5 min at 37°C) to obtain single cell suspensions, washed in MEF medium for enzyme neutralization, filtered through 40 µm cell strainer, and resuspended at a concentration of 1 × 106 cells/ 100 µL in EGM-2/PBS (1:1) solution after centrifugation (200 g, 5 min, room temperature). Cell suspensions were maintained on ice during all staining steps until cell sorting and stained by incubation with monoclonal mouse anti-human CD31-APC (1:20, eBioscience) and CD146-PE (1:10, BD Biosciences) antibodies for 30 min on ice. Cells were sorted simultaneously into four fractions based on CD31 and CD146 expression into 5 mL polypropylene round bottom tubes (BD Biosciences, Cat No. 352063) containing 500 µL of EGM-2 using a special order three-laser BD FACSAria high speed cell sorter (BD Biosciences) and using FACS DIVA software at the Johns Hopkins School of Medicine FACS core facility (Johns Hopkins University, Baltimore, MD). Limited spectral overlap was compensated by the operator using single color stained controls. Cells were diligently transported on ice back to our laboratory after cell sorting.
Dil-acetylated low density lipoprotein (Dil-Ac-LDL) uptake
Our purified putative angioblast populations were tested for their endothelial potential using Dil-acetylated low density lipoprotein (Dil-Ac-LDL) uptake assay (Life Technologies, Cat No. L-3484). Each sorted population (CD31+CD146−, CD31+CD146+, CD31−CD146−, and CD31−CD146+ cells) was plated into fibronectin coated six-well plates (1 to 1.5 × 105 cells/well) and cultured in EGM-2 until they reached 60–70% confluency. Medium was then switched to fresh EGM-2 supplemented with 10 µg/mL Dil-Ac-LDL. Cells were incubated in this medium for 4 h at 37°C before being washed into fresh EGM-2 medium. Dil-Ac-LDL uptaking cells were photographed using an Eclipse Ti-u inverted microscope. Cells were washed in PBS, treated with 0.05% trypsin-EDTA (5 min, 37°C) and processed for flow cytometry analysis similarly to Accutase®-dissociated hEB cells (see “Flow cytometry” Section; “hESC/hiPSC, hEB, and adherent and non-adherent EGM-2 culture-derived hEB cells”).
Immunocytostaining of RUNX1
Day 8 hEB were plated onto fibronectin-coated 12-well plates (0.5 to 1 × 105 cells/well, Greiner Bio-one) and in EGM-2 medium supplemented with 25 ng/mL VEGF for 3–5 days in an incubator (5% CO2, humid atmosphere), as described above. Adherent cells were washed once in PBS and fixed in 1% paraformaldehyde solution (USB Corporation, Cleveland, OH, Cat No. 19943) in PBS for 20–30 min at 4°C. Staining procedure was adapted from (22). Cells were washed twice 5 min at room temperature in Dako wash buffer (Dako, Carpinteria, CA, Cat No. 53006) and incubated for 30 min at room temperature in PBS, 5% goat serum (Sigma Aldrich, Cat No. G9023-10ML), 0.05% Tween20 (Sigma Aldrich, Cat No. 274348) to block unspecific secondary antibody binding. Blocking solution was disposed of and monoclonal mouse anti-human Runx1 antibody (1:50 in blocking solution, Santa Cruz Biotechnology, Santa Cruz, CA, Cat No. SC-101146, Clone DW71) was readily added to the cells and incubated overnight at 4°C. Incubation with Universal Negative Control for Mouse Primary Antibodies (Dako) was used for negative controls. The next day, cells were washed twice for 5 min in Dako buffer and incubated with biotinylated goat anti-mouse antibody (1:500 in blocking solution, Dako, Cat No. E0433) for 1 h at room temperature. After two consecutive washes, cells were incubated with streptavidin-Cy3 (1:500 in PBS, Sigma Aldrich, Cat No. S6402) for 30 min at room temperature. Cells were washed in PBS and nuclear staining was achieved by incubating with DAPI (4′,6-diamidino-2-phenylindole, 1:1,000 in PBS, Life Technologies) for 5 min. All wells were washed prior to observe and photographed using an epifluorescence microscope (Eclipse Ti-u) and NIS-elements BR3.1 software (Nikon Instruments).
Statistical Evaluation
Data are summarized as average ± standard deviation. Statistical evaluation was performed using two-tailed Student’s t-test. P values are indicated in the figure or in the figure legends.
Results
Optimal Hemato-Vascular Differentiation of hiPSC Required Rigorous Prevention of Spontaneous Differentiation
Although fibroblast-derived hiPSC lines can be routinely cultured in standard hESC medium supplemented with 4 ng/mL FGF2 (2), these lines often displayed a higher frequency of spontaneous differentiation in comparison to standard hESC lines grown in our laboratory. Thus, all hemato-endothelial differentiation experiments were initiated by first assuring we started with only the highest quality of undifferentiated pluripotent stem cell culture. hiPSC colonies with poor differentiated morphology were manually depleted from cultures with a micropipette under a phase contrast microscope in a laminar flow hood, and the remainder was enzymatically passaged by collagenase type IV. Alternatively, undifferentiated colonies were manually dissected, and subcloned onto fresh (after overnight seeding) MEF feeders (Supporting Information Fig. 1A). If necessary, high quality undifferentiated hiPSC subcultures were further stabilized with increased concentrations of FGF2 (e.g., 10–20 ng/mL) than normally required for standard hESC culture (e.g., 4–10 ng/mL). Pluripotency marker expression was regularly checked by flow cytometry and only under these rigorous conditions, fibroblast-hiPSC lines with >90%SSEA4+ TRA-81+ OCT-4+ expression could be routinely maintained prior to hEB differentiation (Supporting Information Fig. S1B).
Figure 1. Hemato-vascular differentiation of hESC and fibroblast-iPSC using a serum free hEB system supplemented with BMP4, VEGF, FGF2, and heparan sulfate (BVF2H).
(A) Phase contrast pictures of hEB from H9 and IMR90-4 at day 3 of hEB differentiation (first 2 rows). Day 8 hEBs (bottom 2 rows) are larger and become cystic. The scale bars represent 100 µm. (B) Flow cytometry analysis of dissociated day 10 H9, IMR90-1, and IMR90-4 hEB cells demonstrates similar expressions and percentages of CD143/ACE, CD34, CD31, and CD146. Shown are representative experiments performed at least three times. (C) Flow cytometry analysis of hESC and fibroblast-derived hiPSC (FibroiPSC) and the average percentages of cells with surface expression of CD34, CD143/ACE, CD31, CD146, KDR (VEGF receptor 2, VEGFR2), and CD133. Number of times experiments were performed and P values are indicated.
Hemato-Endothelial Marker Expression on Differentiated hEB was Comparable Between Fibroblast-hiPSC and hESC
We previously demonstrated that mesodermal hemato-endothelial (MHE) and hemangioblastic progenitors sequentially emerge from hESC between 6 and 10 days after hEB differentiation in serum-free medium supplemented with BMP4, VEGF, and FGF2/heparan sulfate (BVF2H) (9, 10) in a manner resembling YS-hematopoiesis of the human embryo. To evaluate the comparative kinetics of hiPSC and hESC hemato-endothelial lineage commitment in our hEB-based system, we tested the differentiation capacities of two fibroblast-derived hiPSC lines, IMR90-1 and IMR90-4, which were previously reported to display diminished hemato-endothelial differentiation potential relative to hESC lines (18, 19). We quantitatively compared the hemo-vascular hEB differentiation efficiency of these hiPSC lines to the hESC line H9 (Fig. 1), which we and others have previously shown exhibits vigorous hemato-endothelial differentiation capacity in both OP9 and hEB differentiation systems (9, 10). Single cell-dissociated hEB cells from all three cell lines were analyzed by flow cytometry analysis at various time points during differentiation for surface marker expression of hemato-endothelial (CD34, CD31) and hemangioblastic (CD143/ACE) antigens (10). Both hESC and hiPSC showed similar kinetics (Fig. 2). A kinetic flow cytometry analysis of hEB cells during differentiation revealed that surface expression of TRA-1-60, TRA-1-81 pluripotency markers rapidly decreased between days 1–5 (Fig. 2). However, surface expression of SSEA4 diminished less rapidly as BVF2H differentiation progressed, with more than 80% of hEB cells still expressing this antigen at day 10 of differentiation. Overall, the kinetics, frequencies, and expressions of these surface markers were relatively similar between hESC and fibroblast-iPSC in this optimized differentiation protocol, although IMR90-4 hiPSC differentiation was more consistently comparable to H9 hESC than IMR90-1. We also noted that surface expression of the mesenchymal-mesodermal marker KDR was abundantly expressed in undifferentiated hESC. Interestingly, surface expression of CD146, a known marker of mesenchymal-pericytic lineage was detected in undifferentiated hESC but not in fibroblast-iPSC (Fig. 2).
Figure 2. Kinetics of hemato-endothelial surface marker expression of differentiated H9, IMR90-1 (IMR-1) and IMR90-4 (IMR-4) hEB cells.
Using our standard hEB differentiation protocol, day 1, 3, 6, 8, 10, 13, 15, and 20 hEBs were harvested, enzymatically digested to single cells, and analyzed for the expressions of SSEA4, TRA-1-60, TRA-1-81, CD143/ACE, CD146, KDR (VEGF receptor 2, VEGFR2), CD34, and CD31.
CD31 and CD146 Identified a Putative Vascular-Pericytic Population
To further identify mesodermal progenitors generated in this system, we next analyzed the hemato-endothelial population generated in EGM-2 culture. We first subdivided CD31+ hEB cells by co-expression of CD146, which has been demonstrated to identify pericyte/perivascular cells (23). All four populations (CD31+CD146−, CD31+CD146+, CD31−CD146−, and CD31−CD146+ cells) were isolated and analyzed for in vitro endothelial functionality (Fig. 3A). CD31+CD146+ cells possessed the highest in vitro uptake of Dil-Ac-LDL among the sorted populations, thus demonstrating the most robust endothelial potential (Fig. 3B).
Figure 3. Endothelial differentiation of hESC and hiPSC.
(A) Day 8 hEB were disaggregated using collagenase type-IV and plated onto the fibronectin coated plates in EGM-2 medium. Day 4 EGM-2-cultured hEB cells were analyzed and purified by FACS for surface expression of CD31 and CD146. (B) Day 8 hEB cells differentiated in EGM-2 medium were FACS-purified into four populations (CD31+CD146−, CD31+CD146+, CD31−CD146−, and CD31−CD146+ cells), and expanded for additional 7—12 days prior to assay of Dil-Acetylated-LDL (Dil-Ac-LDL) uptake to demonstrate endothelial cell function. Representative picture of Dil-Ac-LDL uptaking cells of CD31+CD146+ cells (Left) and the merged image of phase contrast picture (Right) derived from IMR90-4. Scale bars represent 100 µm. Quantitative comparisons of expression of Dil-Ac-LDL uptaking cells from FACS purified four populations (bottom panel). (C) Day 8 hEB clumps differentiated from IMR90-4 generated hematopoietic cobblestones that emerged directly from adherent endothelial layers. Shown are averaged experiments performed three to five times with n, standard deviation, and P values indicated for the values of the CD31+CD146+ group compared to the other three categories of sorted populations (* = P < 0.05 for fibro-iPSC; ** = P < 0.05 for hESC; NS = comparison was not significant).
Adherent BVF2H-Differentiated hEB Cells Simultaneously Generated Progenitors with Vascular and Hematopoietic Phenotypes in Endothelial EGM-2 Medium
To enhance the expansion and differentiation of hemangioblasts in our hEB-based differentiation system, we modified our BVF2H differentiation protocol by including a subsequent adherent differentiation step in endothelial culture conditions (10). This was accomplished with defined endothelial medium (EGM-2) to further expand large numbers of hEB-derived hemato-endothelial progenitors in an adherent two-dimensional culture system. Since day 8 hEB contained mesodermal and hemangioblasts in our previously described serum-free BVF2H differentiation system (10), disaggregated hEB cells from this differentiation time point were cultured onto fibronectin-coated plates in EGM-2. This culture system robustly produced adherent, heterogeneous populations of hEB-derived cells with endothelial, hematopoietic, stromal, perivascular, and undifferentiated morphologies. Moreover, we routinely observed the emergence of adherent hematopoietic clusters with round, cobblestone morphology arising from cells with endothelial morphology (15, 24) (Fig. 3C, Supporting Information Fig. S3). This was followed by the emergence of viable floating cells expressing the hematopoietic progenitor markers CD34, CD45, and CD143/ACE (Fig. 4A). These hematopoietic colonies expressed the RUNX1, transcription factor, which is critical for generating definitive hematopoietic cells from hemogenic endothelium (25, 26) (Supporting Information Fig. S4). These floating cells were plated in hematopoietic methylcellulose medium, and were demonstrated to possess higher hematopoietic progenitor frequency than hEB cells at the same differentiation stage (Fig. 4B).
Adherent EGM-2 Culture of BVF2H-differentiated hEB Cells Generated Augmented Frequencies of Multipotent CD34+CD45+ Hematopoietic Progenitors
To evaluate the efficiency of hematopoietic progenitors that emerged in EGM-2 culture conditions, floating cells were analyzed by flow cytometry analysis at sequential time points for hemato-endothelial markers. These analyses showed that CD34+CD45+ hematopoietic progenitors emerged from H9 and hiPSC in EGM-2 cultures ~1 week following a wave of CD143+CD34+ putative hemangioblasts (pre-hematopoietic progenitors). To evaluate the hematopoietic progenitor potential of these floating cells, hEB cells from day 10 hEB, or floating cells of 3, 4, or 5 additional days in EGM-2 culture that started from day 8 EB were plated in methylcellulose medium and enumerated for CFU activity. These experiments revealed that in comparison to day 10 hEB cells in native hEB, floating hematopoietic cells from EGM-2 cultures contained at least three-fold greater frequencies of hematopoietic colonies, including multipotent mixed erythro-myeloid CFU (Fig. 4B).
Interestingly, in contrast to previous reports (18, 20), under these conditions IMR90-1 and IMR90-4 hiPSC both generated erythroid and myeloid CFU colonies that were comparable or slightly greater than H9 hESC (Fig. 4B). Fibroblast-iPSC generated higher numbers of erythroid cells or CFU-e compared to H9 produce higher numbers of BFU-e and mixed colonies. Notably, the phenotype of hematopoietic colonies suggested embryonic and not adult type definitive hematopoiesis, as erythroid colonies expressed embryonic (Hb-ε) and fetal (Hb-F) but not adult beta hemoglobins (HbA) (Fig. 4C).
Supplementation of EGM-2 with Additional Hematopoietic Growth Factors Directed the Further Lineage Commitment of Emerging Hematopoietic Progenitors
To further enhance hematopoietic progenitor generation in this novel system, cultures were supplemented further with additional hematopoietic growth factors at hEB and EGM-2 differentiation stages. For example, when Thrombopoietin (TPO) and IL6 were both added to either the hEB differentiation stage (from day 3 to 8), or during the EGM-2 culture period thereafter, slightly higher numbers of floating CD34+CD45+ were observed from IMR90-1 and IMR90-4 cell line (Fig. 5A). Addition of TPO and EPO into the EGM-2 medium generated robust erythropoiesis [i.e., CD45−CD71+ and a higher percentage of glycophorin A (CD235a+ cells] compared to EMG2 with simple VEGF supplementation [CD45+CD71+ and minor glycophorin A (CD235a+ cells]. These results suggested that supplementing EGM-2 culture system with lineage-directing hematopoietic growth factors provided a highly efficient means of producing large quantities of lineage-committed erythroid, myeloid, and megakaryocytic progeny (Fig. 5B).
Figure 5. Supplementation of adherent EGM-2 hEB cultures with additional hematopoietic growth factors.
(A) Effects of supplementation with TPO and IL6 during day 3 to 8 of BMP4, VEGF, FGF2, and heparan sulfate (BVF2H) hEB differentiation, and addition of TPO during EGM-2 culture (i.e., day 8 hEB followed by an additional 3, 4, or 5 days in EGM-2 culture). Supplementation with hematopoietic growth factors altered the production of CD34, CD143/ACE, and CD45 expressing hEB cells. The addition of TPO and IL6 during hEB BVF2H differentiation, and the addition of TPO during EGM-2 culture generated the highest percentages of CD34+CD45+ hEB cells in IMR90-4 cells from day 8 of hEB plus day 5 of EGM-2 culture (bottom graph, far right). (B) Day 8 hEB with addition of day 8 in EGM-2 culture supplemented with VEGF (25 ng/mL) of IMR90-1 cells in expression of CD45, CD71, and glycophorin A (a). Addition of TPO and erythropoietin (EPO) (25 ng/mL each) to the EGM-2 medium supplemented with VEGF (25 ng/mL) directed hematopoietic cells to differentiate to the erythroid lineage (b).
Discussion
The human blood-forming system develops by a succession of dynamic events during embryonic life, and emerges from at least two independent sites: the extraembryonic YS and the AGM (8, 27). In both locations, de novo hematopoiesis involves the intimate liaison of blood-forming and angiogenic cells. However, although hematopoietic and endothelial lineages develop in YS blood islands in synchrony from a common bipotent short-lived progenitor (aka the hemangioblast) (9, 10), intraembryonic blood cells bud off of a pool of already established endothelial cells, the hemogenic endothelium (11–14).
We previously described a pluripotent stem cell model that recapitulates the human YS-like origins of embryonic hemato-endothelial development (9). Hematopoiesis arose from CD45-negative MHE progenitors that give rise to CD143/ACE+ hemangioblast progenitors, followed by sequential YS-like primitive and definitive hematopoietic waves. We delineated the kinetics for emergence of these hemangioblastic progenitors responsible for these two waves (10). Thus, our hEB-based differentiation system captures the earliest developmental steps in human hematopoietic genesis (28, 29).
Recently, this pluripotent stem cell model was validated with human embryo studies. Primitive pre-AGM mesodermal ancestors of the human intraembryonic hematopoietic system were identified in the splanchnopleura (Sp) as CD143/ACE, expressing cells (30). ACE, a key regulator of blood pressure and major component of the renin–angiotensin system (31), and also marks human blood-forming cells in the YS (hemangioblasts), hemogenic endothelium (AGM), fetal liver, and both fetal and adult bone marrow HSC (32). In the human Sp, CD143+ hematopoietic-competent cells (identified with the BB9 mAb) coexpressed OCT-4 and SSEA-1 (30), two markers of human primordial germ cells (PGC), which may indicate a possible affiliation, or at least convergence of developmental pathways. OCT-4 and SSEA-1 co-expression defines mouse ESC (but not human, which do not express SSEA-1). It is also noteworthy that only mouse ESC have thus far demonstrated the capacity to generate definitive adult hematopoietic lineages in vitro. To investigate the complex developmental relationship of blood-forming and endothelial precursors during human embryonic life, we and others have developed surrogate hESC and hiPSC in vitro differentiation systems, which tentatively mimic either YS (9, 10) or AGM hematopoiesis (7, 16, 17).
In the present work, we further improved this hEB-based hemato-endothelial differentiation protocol, and modified our developmental approach to obtain AGM-like mesodermal endothelial intermediates. We rigorously defined the minimal culture conditions to promote the emergence of mesodermal precursors from hEB, which were then guided toward endothelial commitment by adherent low-density culture in endothelial medium. The initial loss of pluripotency marker expression (TRA-1-60, TRA-1-81, SSEA4) was accompanied by the acquisition of a mesodermal phenotype (KDR, CD133, CD34, CD146, and CD31), and was highlighted by a peak of CD143/ACE expression at the 8th day of differentiation. The day 8 time point may be analogous to the in vivo emergence of pre-AGM Sp OCT4+ ACE+ cells. The subsequent culture step in EGM-2 on fibronectin gave rise to multiple adherent stromal-vascular cells, distinctive both by morphology and marker expression of the acquisition of stromal, perivascular, or endothelial phenotypes. Of particular interest, adherent round, cobblestone RUNX1+ hematopoietic cell clusters emerged systematically from endothelial-shaped cell colonies, which directly released mass quantities of multipotent CD34+CD41+CD45+CD143+ progenitors. This AGM-like hemogenic endothelium expressed RUNX1 and CD41, two markers indicative of definitive hematopoiesis potential in the mouse. However, the lack of acquisition of adult-type hemoglobins during erythropoiesis highlights the challenges yet to be overcome for generating adult-type human hematopoiesis. Considering the PGC-like phenotype of pre-AGM Sp hematopoietic ancestors (30) and the recently presumed post-implantation epiblast-like phenotype of hESC, one possibility is that human pluripotent stem cells may simply not be able per se to efficiently generate definitive adult type blood cells using contemporaneous hESC culture conditions. In contrast, murine pluripotent stem cells harbor an inner cell mass phenotype and naïve state, which may explain their propensity to generate mature blood lineages. Alternatively, blood-forming cells follow a journey through successive niches (colonization of fetal liver and bone marrow) to mature, receiving niche specific signals that must be taken into account for generating in vitro human adult-type blood.
A major factor impacting pluripotent stem cell differentiation models can be attributed to the hESC/hiPSC undifferentiated expansion stringency, as well as the robustness of the used differentiation system. We demonstrated that under rigorous culture conditions, fibroblast-hiPSC lines can preserve their pluripotency state similarly to hESC, without spontaneous differentiation, which is an essential prerequisite for efficient subsequent differentiation. Although several recent studies pointed out that hiPSC do not produce hemato-endothelial progeny in a manner that is quantitatively and qualitatively comparable to hESC (18, 33), we demonstrated here that using our robust and optimized system, fibroblast-iPSC can support simultaneous hematopoiesis and vasculogenesis, at levels comparable to an hESC line (H9; WA09) with robust hemato-endothelial capacity. However, technical variability among laboratories and the inherent efficiency of the multiple differentiation protocols (e.g., embryoid body vs. stromal coculture) may still constrain the pluripotent state (e.g., MEF vs. feeder-free monolayer) of hESC and hiPSC.
Additionally, while some fibroblast-hiPSC differentiation limitations may be overcome using the optimized culture conditions we described herein, their reported lower differentiation efficiency may also be partially attributed to retention of somatic donor epigenetic memory (34). Indeed, while recent studies pointed out aberrant or incomplete epigenomic reprogramming in hiPSC (35–40), several reprogrammed cell types have been shown to maintain an enhanced differentiation potential toward the lineage they were derived from (41–45). Extended passages did not completely extinguish this limitation for hiPSC clones or allow them to recover an hESC-like epigenomic phenotype (42). Although residual epigenetic marks may alter the differentiation potential of iPSC lines, we show here that such limitations may be at least partially overcome by refining the expansion and differentiation method.
Recent innovative studies have similarly identified multipotent mesoderm vasculogenic precursors, (46, 47) using similar hESC/hiPSC–based methodologies. Although both studies showed the simultaneous emergence of mesenchymal/pericyte and endothelial lineages from human pluripotent cells, Vodyanik et al. demonstrated that both mesenchymal/pericyte (CD146+ CD31− CD45−) and endothelial (CD31) progenies were derive from a common precursor, the “mesenchymoangioblast.” This bipotent progenitor exhibits a mesenchymal (CD105+CD73+CD90+) pericytic (CD140a+CD146+) nonendothelial nor hematopoietic (CD31−CD43−CD45−) phenotype. The mesenchymoangioblast may represent a common progenitor to mesenchymal and endothelial lineages, which might give rise to hemogenic endothelium from which in turn emerge hematopoietic progenitors (48, 49). Mesenchymogenic cells have been believed to arise from both the neural crest and the mesoderm (50). Noticeably, mesoangioblasts, a primitive CD34+ population of angiogenic and mesenchymal progenitors, emerges also from the mouse DA (51). Mesoangioblasts are believed to be the ancestors of postnatal multipotent pericytes, which have been shown to be themselves precursors of fetal and adult mesenchymal stem cells (MSC) (52). Primitive MSC have been proposed to similarly express CD34 based on in vitro models (53, 54), a phenotype shared in vivo by some rare adult bone marrow mesenchymal progenitors (55, 56) but also larger subsets of adipose pericytes (57). Further studies are required to investigate in vitro and in vivo the hierarchy of mesenchymal/pericytic, angiogenic and hematopoietic lineages, and kinetics of divergence, although our system also supports the simultaneous growth of pericyte/mesenchymallike cells (data not shown).
Finally, the isolation of hemangioblasts from hiPSC may provide new opportunities for generating therapeutically relevant autologous transplantable HSC. However, the complex developmental signaling pathways and environments that exist in vivo will need to be recapitulated to further mature these hESC-derived primitive progenitors into adult blood cells before they can be practically used for treatment of hematological disorders.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Karan Verma for technical support, and Ada Tam and Lee Blosser at the Johns Hopkins FACS core facility for cell sorting and technical advice on flow cytometry analysis.
Grant sponsor: NIH; Grant numbers: 1U01HL099775 and U01HL100397; Grant sponsor: Maryland Stem Cell Research Funds; Grant numbers: MSCRF 90039210 and MSCRF II-0008-00
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lengerke C, Daley GQ. Disease models from pluripotent stem cells. Annals of the New York Academy of Sciences. 2009;1176:191–196. doi: 10.1111/j.1749-6632.2009.04962.x. [DOI] [PubMed] [Google Scholar]
- 5.Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells—Opportunities for disease modelling and drug discovery. Nature reviews. Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- 6.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwa A, Umeda K, Chang H, Saito M, Okita K, Takahashi K, Nakagawa M, Yamanaka S, Nakahata T, Heike T. Orderly hematopoietic development of induced pluripotent stem cells via Flk-1(1) hemoangiogenic progenitors. J Cell Physiol. 2009;221:367–377. doi: 10.1002/jcp.21864. [DOI] [PubMed] [Google Scholar]
- 8.Zambidis ET, Sinka L, Tavian M, Jokubaitis V, Park TS, Simmons P, Peault B. Emergence of human angiohematopoietic cells in normal development and from cultured embryonic stem cells. Ann NYAcad Sci. 2007;1106:223–232. doi: 10.1196/annals.1392.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, Pryzhkova M, Peault B. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 12.Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B. Aorta-associated CD341 hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- 13.de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 14.Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 15.Park TS, Burridge P. E. T. Z. Chapter 24: Generation of Multipotent CD34+ CD45+ Hematopoietic progenitors from human induced pluripotent stem cells. In: Marton PJ, editor. Lineage-Specific Differentiation of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells. Springer Protocol Handbook; 2011. [Google Scholar]
- 16.Wang C, Tang X, Sun X, Miao Z, Lv Y, Yang Y, Zhang H, Zhang P, Liu Y, Du Y, et al. TGFbeta inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res. 2012;22:194–207. doi: 10.1038/cr.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joo HJ, Kim H, Park SW, Cho HJ, Kim HS, Lim DS, Chung HM, Kim I, Han YM, Koh GY. Angiopoietin-1 promotes endothelial differentiation from embryonic stem cells and induced pluripotent stem cells. Blood. 2011;118:2094–20104. doi: 10.1182/blood-2010-12-323907. [DOI] [PubMed] [Google Scholar]
- 18.Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, Kim KS, Lanza R. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2009;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 19.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melichar H, Li O, Ross J, Haber H, Cado D, Nolla H, Robey EA, Winoto A. Comparative study of hematopoietic differentiation between human embryonic stem cell lines. PLoS One. 2011;6:e19854. doi: 10.1371/journal.pone.0019854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Peault B. Perivascular multipotent progenitor cells in human organs. Ann NY Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sroczynska P, Lancrin C, Kouskoff V, Lacaud G. The differential activities of Runx1 promoters define milestones during embryonic hematopoiesis. Blood. 2009;114:5279–5289. doi: 10.1182/blood-2009-05-222307. [DOI] [PubMed] [Google Scholar]
- 26.Friedman AD. Cell cycle and developmental control of hematopoiesis by Runx1. J Cell Physiol. 2009;219:520–524. doi: 10.1002/jcp.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peault B, Tavian M. Hematopoietic stem cell emergence in the human embryo and fetus. Ann NY Acad Sci. 2003;996:132–140. doi: 10.1111/j.1749-6632.2003.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 28.Bollerot K, Pouget C, Jaffredo T. The embryonic origins of hematopoietic stem cells: A tale of hemangioblast and hemogenic endothelium. APMIS. 2005;113:790–803. doi: 10.1111/j.1600-0463.2005.apm_317.x. [DOI] [PubMed] [Google Scholar]
- 29.Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C, de Bruijn M. From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp Hematol. 2005;33:1029–1040. doi: 10.1016/j.exphem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Sinka L, Biasch K, Khazaal I, Peault B, Tavian M. Angiotensin-converting enzyme (CD143) specifies emerging lympho-hematopoietic progenitors in the human embryo. Blood. 2012;119:3712–3723. doi: 10.1182/blood-2010-11-314781. [DOI] [PubMed] [Google Scholar]
- 31.Park TS, Zambidis ET. A role for the renin-angiotensin system in hematopoiesis. Haematologica. 2009;94:745–747. doi: 10.3324/haematol.2009.006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jokubaitis VJ, Sinka L, Driessen R, Whitty G, Haylock DN, Bertoncello I, Smith I, Peault B, Tavian M, Simmons PJ. Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal, and adult hematopoietic tissues. Blood. 2008;111:4055–4063. doi: 10.1182/blood-2007-05-091710. [DOI] [PubMed] [Google Scholar]
- 33.Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, Stewart R, Thomson JA, Slukvin II. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011;13:541–549. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, Ng S, Sourour M, Hamalainen R, Olsson C, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 36.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, Plath K, Lowry WE, Benvenisty N. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizzi R, Di Pasquale E, Portararo P, Papait R, Cattaneo P, Latronico MV, Altomare C, Sala L, Zaza A, Hirsch E, et al. Post-natal cardiomyocytes can generate iPS cells with an enhanced capacity toward cardiomyogenic re-differentation. Cell Death Differ. 2012;19:1162–1174. doi: 10.1038/cdd.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Hongguang H, Loh YH, Aryee MJ, Lensch MW, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2012;29:117–119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaff N, Lachmann N, Kohlscheen S, Sgodda M, Arauzo-Bravo MJ, Greber B, Kues W, Glage S, Baum C, Niemann H, et al. Efficient hematopoietic redifferentiation of induced pluripotent stem cells derived from primitive murine bone marrow cells. Stem Cells Dev. 2012;21:689–701. doi: 10.1089/scd.2011.0010. [DOI] [PubMed] [Google Scholar]
- 44.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential line-age-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Quattrocelli M, Palazzolo G, Floris G, Schoffski P, Anastasia L, Orlacchio A, Vandendriessche T, Chuah MK, Cossu G, Verfaillie C, et al. Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs. J Pathol. 2011;223:593–603. doi: 10.1002/path.2845. [DOI] [PubMed] [Google Scholar]
- 46.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm- derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluri-potent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 48.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 50.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cossu G, Bianco P. Mesoangioblasts—Vascular progenitors for extravascular mesodermal tissues. Curr Opinion Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Crisan M. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Kopher RA, Penchev VR, Islam MS, Hill KL, Khosla S, Kaufman DS. Human embryonic stem cell-derived CD341 cells function as MSC progenitor cells. Bone. 2010;47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbet R, Peiffer I, Hatzfeld A, Charbord P, Hatzfeld JA. Comparison of gene expression in human embryonic stem cells, hESC-derived mesenchymal stem cells and human mesenchymal stem cells. Stem Cells Int. 2011;2011:368192. doi: 10.4061/2011/368192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser S, Hackanson B, Follo M, Mehlhorn A, Geiger K, Ihorst G, Kapp U. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. 2007;9:439–450. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 56.Simmons DJ, Seitz P, Kidder L, Klein GL, Waeltz M, Gundberg CM, Tabuchi C, Yang C, Zhang RW. Partial characterization of rat marrow stromal cells. Calcified Tissue International. 1991;48:326–334. doi: 10.1007/BF02556152. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerlin L, Donnenberg VS, Donnenberg AD. Pericytes: A universal adult tissue stem cell? Cytometry Part A. 2012;81A:12–14. doi: 10.1002/cyto.a.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







