Abstract
Objective
To evaluate visual acuity outcomes after cataract surgery in persons with varying severity of age-related macular degeneration (AMD).
Design
Cohort study.
Participants
A total of 1232 eyes of 793 participants who underwent cataract surgery during the Age-Related Eye Disease Study 2 (AREDS2), a prospective, multicenter, randomized controlled trial of nutritional supplements for treatment of AMD.
Methods
Preoperative and postoperative characteristics of participants who underwent cataract extraction during the 5 year trial were analyzed. Both clinical data and standardized red-reflex lens and fundus photographs were obtained at baseline and annually. Photographs were graded by a centralized reading center for cortical and posterior subcapsular lens opacities and for AMD severity. Cataract surgery was documented at annual study visits or by history during the 6 month telephone calls. Analyses were conducted using multivariate repeated-measures regression.
Main Outcome Measures
Change in best-corrected visual acuity (BCVA) after cataract surgery compared with preoperative BCVA.
Results
Adjusting for age at time of surgery, gender, interval between preoperative and postoperative visits, and type and severity of cataract, the mean changes in visual acuity were as follows: eyes with mild AMD (n=30) gained 11.2 letters (95% confidence interval (CI), 6.9-15.5), eyes with moderate AMD (n=346) gained 11.1 letters (95% CI, 9.1-13.2), eyes with severe AMD (n=462) gained 8.7 letters (95% CI, 6.7-10.7), eyes with non-central geographic atrophy (n=70) gained 8.9 letters (95% CI, 5.8-12.1), and eyes with advanced AMD (central geographic atrophy and/or neovascular) AMD (n=324) gained 6.8 letters (95% CI, 4.9-8.8). The visual acuity gain across all AMD severity groups was statistically significant from pre-operative state (P<0.0001).
Conclusions
Mean visual acuities improved significantly after cataract surgery across varying degrees of AMD severity.
Age-related macular degeneration (AMD) and cataracts are significant causes of visual impairment in the United States. AMD affects over 1.75 million individuals in the United States, and it is estimated that this number will increase to 3 million by the year 2020.1 Approximately 20.5 million Americans 40 years or older have cataracts, and this number is estimated to increase to 30.1 million by 2020.2 The incidence of cataracts and AMD will continue to rise with the growing elderly population, resulting in visual disability, a decrease in quality of life, and an increase in the risk of falls, fractures, depression, and mortality.3,4
The relationship between cataract surgery and AMD progression is fraught with controversy, with some studies showing that cataract surgery accelerates the progression of AMD5-7 while others revealing no such correlation.8-11 Two large population-based studies, the Blue Mountain Eye Study12 and the Beaver Dam Eye Study13,14 reported an association between cataract surgery and accelerated AMD progression. A more recent study comparing visual acuity outcomes after cataract surgery between healthy and AMD eyes showed that patients with clinical signs of AMD had worse visual outcomes than those without clinical evidence of AMD, but that over 75% of AMD patients had better visual acuity 10 years after surgery compared with preoperatively.15 Results from the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization (ANCHOR) and the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) phase 3 trials showed that cataract surgery was safe in patients with AMD, including eyes treated with ranibizumab, and that visual acuity improvement was generally observed.16
The Age-Related Eye Disease Study (AREDS), a multi-center trial of oral supplementation containing vitamin C, vitamin E, beta carotene, zinc, and copper (the AREDS formulation) that reduced the 5-year risk of developing advanced AMD by 25%,17 did not demonstrate any harmful or beneficial effects on the development or progression of cataracts.18 Furthermore, cataract surgery did not increase the risk of AMD progression19 and showed that patients with varying severity of AMD had improved visual acuity outcomes after surgery.20
Recent results from AREDS2 demonstrated that the addition of lutein and zeaxanthin to the AREDS formulation did not significantly affect the rates of cataract surgery or moderate vision loss.22 The addition of lutein/zeaxanthin to the AREDS formulation did have an incremental beneficial effect on reducing the risk of progression to advanced AMD in the main effects analyses, especially when lutein/zeaxanthin was substituted for the beta-carotene in original AREDS formulation. However, the addition of omega-3 polyunsaturated fatty acids (specifically docosahexaenoic acid (DHA) (350 mg) and eicosapentaenoic acid (EPA) (650 mg), had no effect on the progression to advanced AMD.21 The objective of this study was to evaluate visual acuity outcomes after cataract surgery among participants in the AREDS2 cohort.
METHODS
Participants and Study Design
Details of the AREDS2 study design and methods are reported elsewhere23 and briefly summarized here. A total of 4203 participants, aged 50 to 85 years, were enrolled into the study from October 2006 to September 2008 at 82 retinal specialty clinics across the United States. Participants were determined to be at high risk for developing advanced AMD, defined as either having bilateral large drusen or nonfoveal geographic atrophy, or large drusen or nonfoveal geographic atrophy in 1 eye and advanced AMD (neovascular AMD or central geographic atrophy) in the fellow eye. Institutional review board approval was obtained at each clinical site, and participants signed informed consent for the study. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Participants were randomly assigned to 4 groups: 1) placebo; 2) lutein (10mg)/ zeaxanthin (2 mg); 3) DHA/ EPA (1 g total); or 4) lutein plus zeaxanthin and DHA plus EPA. All participants were also offered the AREDS formulation and those who agreed to participate were randomly assigned to 4 groups (all receiving 500mg Vitamin C, 400 IU Vitamin E, and 2mg cupric oxide) and either: 1) 15mg beta-carotene plus 80mg zinc oxide; 2) 80mg zinc oxide; 3) 15mg beta-carotene plus 25 mg zinc oxide; or 4) 25mg zinc oxide.
A comprehensive eye examination, which included assessment of best-corrected visual acuity (BCVA) and red reflex lens and fundus photographs, was obtained at baseline and annually thereafter. Telephone calls were made to each participant 3 months after randomization and every 6 months thereafter in between study visits to obtain information about cataract surgery, AMD treatment, and any adverse events. Photographs were graded for lens opacities and severity of AMD by trained and certified examiners using a standardized protocol at the University of Wisconsin Fundus Photograph Reading Center, Madison, Wisconsin. Fundus photos were graded for drusen characteristics (type, area), pigmentary abnormalities (increased pigment or hypo/depigmentation), geographic atrophy, and presence of abnormalities characteristic of neovascular AMD (retinal pigment epithelial detachment, serous or hemorrhagic sensory retinal detachment, subretinal or subretinal pigment epithelial hemorrhage, subretinal fibrous tissue). In the present study, the AREDS AMD severity scale (AAS)24 was used to classify patients into one of 5 categories: mild AMD (AAS 1-3), moderate AMD (AAS 4-6), severe AMD (AAS 7-8), non-central geographic atrophy (GA) (AAS 9), and advanced AMD (CGA and/or neovascular AMD) (AAS 10-11).
The study ophthalmologist examined the anterior segment using slit lamp biomicroscopy at the annual visit to diagnose or confirm the presence of pseudophakia or aphakia. The severity and progression of cortical and posterior subcapsular cataract (PSC) opacities on the red reflex lens photos and the presence of pseudophakia or aphakia were graded at the reading center. Our analysis only included eyes that had cataract surgery during AREDS2. Eyes that were pseudophakic or aphakic at baseline were excluded from the study.
Lens Opacity Grading
Masked grading of the red reflex lens photographs was performed to assess the severity of cortical and PSC opacities. The data quality for photograph grading has been previously reported,22 showing a 93% agreement for the presence of cortical opacities and 97% agreement for the presence of PSC. The AREDS2 grid25 was digitally overlaid on the fundus photographs for evaluation, and the area of lens involvement in sectors of the grid was used to grade the opacities. Cortical and PSC opacities appear as darkly shaded interruptions of the red-orange fundus reflex, and any lens area that is darkened was considered to be involved. Grading for cortical and PSC opacities was performed for the study visit closest from the patient’s surgery date. The reader estimated the percentage of cortical opacity for the entire visible lens. Eyes were classified into 1 of 3 cortical opacity groups, ignoring PSC opacity:
Group 1: Cortical = 0%
Group 2: Cortical >0% and ≤5%
Group 3: Cortical >5%
The PSC opacities were graded within a 5-mm diameter circle of the central lens. Eyes were classified into 1 of 3 PSC opacity groups, ignoring cortical opacity:
Group 1: Posterior subcapsular = 0%
Group 2: Posterior subcapsular >0% and ≤5%
Group 3: Posterior subcapsular >5%
Nuclear opacities were not included in the analysis because they were not graded by standardized photographs, but rather by clinical slit-lamp examination, which may be subject to considerable variability.
The primary outcome of our analysis was the change in BCVA after cataract surgery as compared to before surgery. The documented BCVA before surgery and first recorded postoperative BCVA were used for analysis. Change in visual acuity was the difference in BCVA score between the closest preoperative and postoperative study visits.
Statistical Analysis
A multivariate model using repeated-measures regression analysis was used. Because some patients contributed both eyes to the analysis, we accounted for the correlation between eyes within a person by specifying a compound symmetry covariance structure. The MIXED procedure of the SAS System (SAS version 9.3; SAS Institute Inc., Cary, NC) was used. Risk factors included in the model were age at the time of cataract surgery, gender, preoperative AMD level, time between assessment of preoperative and postoperative visual acuity, and cataract opacity groups. Nominal statistical significance was set at 0.05.
RESULTS
Of the 8406 eyes (4203 participants) in the AREDS2 study, 3762 eyes (2092 participants) underwent cataract surgery, with 2370 surgeries done prior to enrollment in the study and 1392 undergoing surgery during the study. A total 1232 eyes of 793 patients with required post-operative visual acuity assessments were included in this study. There were 460 (58%) females and 333 (42%) males. Mean age at the time of first surgery was 77.1 years (+6.0, median 78, range: 54-90). These patients (eyes) could have any number of years of follow-up. The mean time between the preoperative visual acuity and surgery was 5.9 ± 3.6 months and the mean time between surgery and the post-operative visual acuity was 7.0 ±3.6 months.
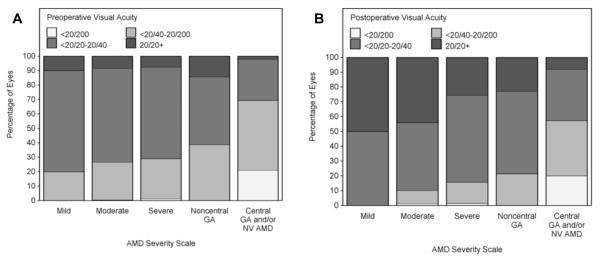
Ocular characteristics of participants who underwent cataract surgery are summarized in Table 1. The preoperative visual acuities are depicted in Figure 1. The mean time from the last recorded preoperative visual acuity to the operative date was 5.9 months (+3.6, median 6.0, interquartile range (IQR): 2.8-8.8), and the mean time from the operative date to postoperative BCVA was 7 months (+3.6, median 7.0, IQR: 4.0-10.1). The mean time between measurement of preoperative and postoperative visual acuities was 12.9 months (+3.1, median 12, IQR: 11.7-13.2).
Table 1.
Ocular Characteristics of Participants Undergoing Cataract Surgery (n = 1232)
| Mild AMD | Moderate AMD |
Severe AMD | Noncentral GA |
Central GA and/or Neovascular AMD |
|
|---|---|---|---|---|---|
| N(%) | 30 (2.4%) | 346 (28.1%) | 462 (37.5%) | 70 (5.7%) | 324 (26.3%) |
| Cortical cataract * | |||||
| Group 1 | 19 (63.3%) | 264 (76.3%) | 341 (73.8%) | 53 (75.7%) | 225 (69.4%) |
| Group 2 | 1 (3.3%) | 41 (11.8%) | 67 (14.5%) | 10 (14.3%) | 47 (14.5%) |
| Group 3 | 10 (33.3%) | 41 (11.8%) | 54 (11.7%) | 7 (10%) | 52 (16%) |
| PSC * | |||||
| Group 1 | 24 (80%) | 296 (85.5%) | 388 (84%) | 59 (84.3%) | 259 (79.9%) |
| Group 2 | 4 (13.3%) | 45 (13%) | 70 (15.2%) | 11 (15.7%) | 50 (15.4%) |
| Group 3 | 2 (6.7%) | 5 (1.4%) | 4 (0.9%) | 0 (0%) | 15 (4.6%) |
AMD = age-related macular degeneration; GA = geographic atrophy; PSC = posterior subcapsular cataract
Group 1: cortical/PSC =0%; Group 2: cortical/PSC >0% and ≤5%; Group 3: cortical/PSC >5%
Figure 1.
Preoperative (A) and postoperative (B) visual acuity of eyes analyzed. Percentages are shown for eyes with varying degrees of preoperative visual acuity and AMD severity. The visual acuity score is represented as Snellen equivalents.
Results of the multivariable repeated-measures regression analysis are shown in Table 2. Adjusting for gender, age at the time of surgery, years between preoperative and postoperative acuity, and cortical and PSC severity groups, a statistically significant mean gain of visual acuity after cataract surgery was observed across all AMD severity groups. Patients with mild AMD gained 11.2 letters (P<0.0001, 95% CI, 6.9-15.5), patients with moderate AMD gained 11.1 letters (P<0.0001, 95% CI, 9.1-13.2), patients with severe AMD gained 8.7 letters (P<0.0001, 95% CI, 6.7-10.7), patients with non-central GA gained 8.9 letters (P<0.0001, 95% CI, 5.8-12.1), and patients with advanced AMD gained 6.8 letters (P<0.0001, 95% CI, 4.9-8.8). There was no statistically significant difference between genders, with females showing a mean visual acuity gain of 9.3 letters (P<0.0001, 95% CI, 7.3-11.3) and males showing a mean gain of 9.4 letters (P<0.0001, 95% CI, 7.4-11.5). In eyes with preoperative visual acuity of 20/40 or worse, a significant gain in visual acuity after cataract surgery was also observed across all AMD severity groups (Table 3).
Table 2.
Adjusted* Gain in Visual Acuity Score after Cataract Surgery at a Mean Postoperative Period of 7 months (n=1233)
| Group | Estimate (Gain of Letters on Visual Acuity Score) |
95% CI | P Value |
|---|---|---|---|
| AMD Severity | |||
| Mild AMD | 11.2 | 6.9-15.5 | <0.0001 |
| Moderate AMD | 11.1 | 9.1-13.2 | <0.0001 |
| Severe AMD | 8.7 | 6.7-10.7 | <0.0001 |
| Noncentral GA | 8.9 | 5.8-12.1 | <0.0001 |
|
Central GA
and/or Neovascular AMD |
6.8 | 4.9-8.8 | <0.0001 |
| Cortical cataract + | |||
| Group 1 | 8.3 | 6.5-10.0 | <0.0001 |
| Group 2 | 8.9 | 6.5-11.4 | <0.0001 |
| Group 3 | 10.9 | 8.4-13.3 | <0.0001 |
| PSC + | |||
| Group 1 | 5.4 | 4.1-6.6 | <0.0001 |
| Group 2 | 7.4 | 5.4-9.4 | <0.0001 |
| Group 3 | 15.3 | 10.8-19.8 | <0.0001 |
AMD = age-related macular degeneration; CI = confidence interval; GA = geographic atrophy; PSC = posterior subcapsular cataract
Adjusted for age at time of surgery, gender, years between preoperative and postoperative acuity, and AMD severity, cortical cataract, or posterior subcapsular cataract
Group 1: cortical/PSC =0%; Group 2: cortical/PSC >0% and ≤5%; Group 3: cortical/PSC >5%
Table 3.
Adjusted* Gain in Visual Acuity Score after Cataract Surgery Based on Preoperative AMD Severity and Lens Opacities in Eyes with Preoperative Visual Acuity of 20/40 or Worse (n=733)
| Group | Estimate (Gain of Letters on Visual Acuity Score) |
95% CI | P Value |
|---|---|---|---|
| AMD Severity | |||
| Mild AMD | 15.0 | 8.2-21.8 | <0.0001 |
| Moderate AMD | 14.3 | 11.6-17.1 | <0.0001 |
| Severe AMD | 9.9 | 7.4-12.3 | <0.0001 |
| Noncentral GA | 10.7 | 6.4-15.1 | <0.0001 |
|
Central GA
and/or Neovascular AMD |
7.1 | 4.8-9.4 | <0.0001 |
| Cortical cataract + | |||
| Group 1 | 10.2 | 7.9-12.5 | <0.0001 |
| Group 2 | 10.5 | 7.4-13.6 | <0.0001 |
| Group 3 | 13.5 | 10.2-16.8 | <0.0001 |
| PSC + | |||
| Group 1 | 8.5 | 6.6-10.4 | <0.0001 |
| Group 2 | 9.8 | 7.1-12.6 | <0.0001 |
| Group 3 | 15.9 | 10.7-21.0 | <0.0001 |
AMD = age-related macular degeneration; CI = confidence interval; GA = geographic atrophy; PSC = posterior subcapsular cataract
Adjusted for age at time of surgery, gender, years between preoperative and postoperative acuity, and AMD severity, cortical cataract, or posterior subcapsular cataract
Group 1: cortical/PSC =0%; Group 2: cortical/PSC >0% and ≤5%; Group 3: cortical/PSC >5%
The postoperative visual acuity as a function of preoperative visual acuity is depicted graphically in Figure 2. After cataract surgery, the proportion of patients improving by more than 10 letters was 36.7% in the mild AMD group, 32.3% in the moderate AMD group, 25.7% in the severe AMD group, 21.4% in the non-central GA group, and 23.5% in the advanced AMD group. The percentage of patients improving by more than 15 letters was 20%, 15%, 12.1%, 14.3%, and 14.5% in the mild, moderate, severe, non-central GA, and advanced AMD groups, respectively (Figure 3).
Figure 2.
Visual acuity scores before and after cataract surgery. Visual acuity scores and corresponding Snellen equivalents are represented in the preoperative (x-axis) and postoperative (y-axis) visits. The diagonal line intersecting the origin represents no change in visual acuity, and the values below the diagonal line represent a decrease in visual acuity.
Figure 3.
Change in visual acuity after cataract surgery with varying AMD severity. Percentage of patients with gain or loss of letters on the logarithm of minimal angle of resolution of visual acuity chart is shown for eyes with mild AMD, moderate AMD, severe AMD, non-central geographic atrophy, and advanced AMD.
After adjusting for covariates, a statistically significant gain in visual acuity in patients with cortical and PSC opacities after surgery was found. The gain in visual acuity increased with increasing severity of cortical or PSC grade. Eyes with no cortical opacity (group 1) had a mean gain of 8.3 letters (P<0.0001, 95% CI, 6.5-10.0), those in group 2 gained 8.9 letters (P<0.0001, 95% CI, 6.5-11.4), and eyes in group 3 gained 10.9 letters (P<0.0001, 95% CI, 8.4-13.3). Eyes with no PSC opacity had a mean gain of 5.4 letters (P<0.0001, 95% CI, 4.1-6.6) after cataract surgery, those in group 2 PSC gained 7.4 letters (P<0.0001, 95% CI, 5.4-9.4), and those in group 3 PSC gained 15.3 letters (P<0.0001, 95% CI, 10.8-19.8). In eyes with preoperative visual acuity of 20/40 or worse, a statistically significant improvement in visual acuity was observed across all cortical and PSC groups (Table 3).
In an analysis that further divided the advanced AMD group into those with CGA only (n=73) and those with neovascular AMD with or without CGA (n=251), eyes with CGA only had a mean gain of 4.4 letters (P=0.005, 95% CI, 1.3-7.5), while those with neovascular AMD had a mean gain of 7.5 letters (P<0.0001, 95% CI, 5.4-9.5). Visual acuity gain between these two groups was not statistically significant (P=0.14).
Because diabetes can affect lens clarity, we analyzed whether diabetes was a significant factor for visual acuity change after surgery. Patients with a history of diabetes (n=107) had a mean gain of 9.0 letters (95% CI, 6.5-11.6), while those without diabetes (n=686) had a mean gain of 9.4 (95% CI, 7.5-11.4). This was not statistically significant between the two groups (P=0.71).
DISCUSSION
This study highlights the visual acuity outcomes of a large cohort of patients who underwent cataract surgery during the AREDS2 study, with analysis using standardized and validated photographic techniques for grading AMD severity and lens opacities, and best-corrected visual acuities at all study visits. This population is unique in that all patients had at least a moderate risk for AMD. After adjusting for covariates, a statistically significant gain in visual acuity after cataract surgery was found among all levels of AMD severity.
Concurrence of AMD and cataract is common among the aging population, and ophthalmologists are often faced with the decision on whether to preform cataract surgery on these patients. Although prior studies have described worsening maculopathy after surgery12-14 our present study, similar to previous results in AREDS,20 demonstrated that visual acuity improved after cataract surgery in patients with intermediate to advanced AMD. We also showed a correlation with higher improvement in visual acuity with increasing severity of cortical and PSC opacities.
Compared to the original AREDS study,20 the AREDS2 cohort showed a greater mean improvement in visual acuity across all AMD severity groups. There may be several possible explanations for this observation. First, baseline vision before cataract surgery was worse in the AREDS2 cohort compared with AREDS participants. Because change in visual acuity was used as our primary outcome measure, patients with worse baseline vision would likely show a greater gain in acuity after surgery. Second, technological advances in cataract surgery techniques over the past decade have reduced complication rates and greatly improved visual outcomes. p Furthermore, the grading for lens opacities differed between the two studies. AREDS used slit-lamp photographs to grade nuclear opacities and retroillumination photographs to grade cortical and PSC opacities. AREDS2 only used red reflex photographs for grading cortical and PSC opacities; slit-lamp photographs were not obtained to evaluate nuclear cataracts.
The advent of anti-VEGF intravitreal injections has revolutionized the treatment of, and improved the visual prognosis for patients with AMD. Anti-VEGF agents were the standard AMD treatment for patients enrolled in the AREDS2 cohort; these therapies were not available when AREDS was being conducted in the 1990’s. Prior to the introduction of anti-VEGF agents, cataract surgery was believed to increase the risk of neovascular hemorrhage and leakage, resulting in worsening visual deterioration. Older treatment modalities, which included argon laser photocoagulation and photodynamic therapy, could, at its very best, only limit vision loss. Significant risk for laser-induced retinal damage, recurrent CNV, and permanent vision loss were not uncommon. In contrast, anti-VEGF agents have been shown to stabilize vision in more than 90% of cases and improve vision in more than 30%.26-28 Rosenfeld et al. showed that cataract surgery was beneficial in patients with neovascular AMD receiving monthly ranibizumab injections during the ANCHOR and MARINA phase 3 trials, with a mean visual acuity improvement of 10.4 letters three months postoperatively.16 Thus the use of these anti-VEGF agents may, at least in part, account for the improvement in visual acuity after cataract surgery seen in neovascular AMD patients.
Strengths of the study include a large, well-defined population, longitudinal patient follow-up, and a standardized protocol for assessing lens opacities and visual acuities. The primary outcome (change in BCVA before and after surgery) of this study is an easily quantifiable measure.
This study has several limitations. The distribution of eyes across the different AMD severity groups was not uniform, ranging from 2.4% of eyes in the mild AMD group to 37.5% of eyes in the severe AMD group. Fixed interval follow-up on all patients was not possible due to the requirement for only annual study visits and long-term follow-up data was available on only a relatively small number of eyes, especially those in the mild AMD group, which may have limited the power for this sub-analysis. This may explain the smaller gain in visual acuity among patients with mild AMD compared to the other AMD groups in the long-term follow-up analysis. There could also have been a selection bias to enroll a select group of patients into the study. The AREDS2 cohort was highly educated, well-nourished, and predominantly white. Issues of greater access to care, threshold for undergoing cataract surgery, and the tendency for healthier behaviors may bias the results of the study. As such, the results of this study may not be generally applicable to all AMD patients. In addition, the primary focus of the AREDS2 study was on retinal outcomes, but we were able to analyze lens outcomes by incorporating red reflex photographs for grading of cortical and PSC opacities. Assessment of nuclear opacities requires specialized slit-lamp photography, which was not readily available in all 82 clinical sites; data on nuclear opacities was incomplete and not used for analysis in this study. We acknowledge that nuclear opacities account for a substantial proportion of overall lens opacities and our results must be interpreted in this context.
In summary, our findings demonstrate that AMD patients achieved a significant improvement in visual acuity after cataract surgery across varying AMD severity. Patients with concurrent visually significant cataract and AMD should not be discouraged from undergoing cataract surgery, as they may benefit from visual improvement after surgery.
Acknowledgments
Financial Support: Supported by the intramural program funds and contracts from the National Eye Institute/National Institutes of Health, Department of Health and Human Services, Bethesda Maryland (contract HHS-N-260-2005-00007-C; ADB contract N01-EY-5-0007) The sponsor and funding organization participated in the design and conduct of the study; data collection, management, analysis and interpretation; and the preparation, review and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: This work was presented at the 2013 Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, Seattle, Washington.
Conflict of interest: Dr. Susan Bressler reported receiving grant and travel support from the EMMES Corporation and the Jaeb Center; receiving Physician-Scientist grant from Research to Prevent Blindness, serving as a consultant for GlaxoSmithKline; receiving grants or grants pending from Allergan, Bausch and Lomb, Bayer Healthcare, Genentech, Lumenis Inc, Notal Vision Ltd, Novartis, Regeneron, Thrombogenics, and Sanofi-Aventis; receiving payment for lectures from providers of continuing medical education materials; and these grants are negotiated and administered by the School of Medicine, which receives the grants through the Office of Research Administration (individual investigators who participate in such sponsored projects are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the projects).
Dr Rosenfeld reported serving as a consultant for Oraya, Novartis, Chengdu Kanghong Biotech, Acucela, Thrombogenics, and Canon; receiving grants or grants pending from Carl Zeiss Meditec, Alexion, Potentia, and GlaxoSmithKline; and receiving payment for lectures from Carl Zeiss Meditec, Allergan, and Topcon.
REFERENCES
- 1.Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Eye Diseases Prevalence Research Group Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 3.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108:1893–900. doi: 10.1016/s0161-6420(01)00754-0. [DOI] [PubMed] [Google Scholar]
- 4.Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012;308:493–501. doi: 10.1001/jama.2012.9014. [DOI] [PubMed] [Google Scholar]
- 5.Pollack A, Marcovich A, Bukelman A, Oliver M. Age-related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology. 1996;103:1546–54. doi: 10.1016/s0161-6420(96)30464-8. [DOI] [PubMed] [Google Scholar]
- 6.Freeman EE, Munoz B, West SK, et al. Is there an association between cataract surgery and age-related macular degeneration? Data from three population-based studies. Am J Ophthalmol. 2003;135:849–56. doi: 10.1016/s0002-9394(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 7.Kaiserman I, Kaiserman N, Elhayany A, Vinker S. Cataract surgery is associated with a higher rate of photodynamic therapy for age-related macular degeneration. Ophthalmology. 2007;114:278–82. doi: 10.1016/j.ophtha.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Sutter FK, Menghini M, Barthelmes D, et al. Is pseudophakia a risk factor for neovascular age-related macular degeneration? Invest Ophthalmol Vis Sci. 2007;48:1472–5. doi: 10.1167/iovs.06-0766. [DOI] [PubMed] [Google Scholar]
- 9.Baatz H, Darawsha R, Ackermann H, et al. Phacoemulsification does not induce neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1079–83. doi: 10.1167/iovs.07-0557. [DOI] [PubMed] [Google Scholar]
- 10.Dong LM, Stark WJ, Jefferys JL, et al. Progression of age-related macular degeneration after cataract surgery. Arch Ophthalmol. 2009;127:1412–9. doi: 10.1001/archophthalmol.2009.152. [DOI] [PubMed] [Google Scholar]
- 11.Hooper CY, Lamoureux EL, Lim L, et al. Cataract surgery in high-risk age-related macular degeneration: a randomized controlled trial. Clin Experiment Ophthalmol. 2009;37:570–6. doi: 10.1111/j.1442-9071.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 12.Cugati S, Mitchell P, Rochtchina E, et al. Cataract surgery and the 10-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2006;113:2020–5. doi: 10.1016/j.ophtha.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Wong TY, et al. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2002;120:1551–8. doi: 10.1001/archopht.120.11.1551. [DOI] [PubMed] [Google Scholar]
- 14.Klein BE, Howard KP, Lee KE, et al. The relationship of cataract and cataract extraction to age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2012;119:1628–33. doi: 10.1016/j.ophtha.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mönestam E, Lundqvist B. Long-term visual outcome after cataract surgery: comparison of healthy eyes and eyes with age-related macular degeneration. J Cataract Refract Surg. 2012;38:409–14. doi: 10.1016/j.jcrs.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, Shapiro H, Ehrlich JS, Wong P, MARINA and ANCHOR Study Groups Cataract surgery in ranibizumab-treated patients with neovascular age-related macular degeneration from the phase 3 ANCHOR and MARINA trials. Am J Ophthalmol. 2011;152:793–8. doi: 10.1016/j.ajo.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–52. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew EY, Sperduto RD, Milton RC, et al. Risk of advanced age-related macular degeneration after cataract surgery in the Age-Related Eye Disease Study: AREDS report 25. Ophthalmology. 2009;116:297–303. doi: 10.1016/j.ophtha.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forooghian F, Agrón E, Clemons TE, et al. AREDS Research Group Visual acuity outcomes after cataract surgery in patients with age-related macular degeneration: Age-Related Eye Disease Study report no. 27. Ophthalmology. 2009;116:2093–100. doi: 10.1016/j.ophtha.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study 2 (AREDS2) Research Group Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013;131:843–50. doi: 10.1001/jamaophthalmol.2013.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AREDS2 Research Group. Chew EY, Clemons T, SanGiovanni JP, et al. The Age-related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1) Ophthalmology. 2012;119:2282–9. doi: 10.1016/j.ophtha.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danis RP, Domalpally A, Chew EY, et al. AREDS2 Study Group Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2) (AREDS2 report number 2) Invest Ophthalmol Vis Sci. 2013;54:4548–54. doi: 10.1167/iovs.13-11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–7. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 28.Regillo CD, Brown DM, Abraham P, et al. PIER Study Group Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]