Abstract
Background:
Essential oils (EOs) have been claimed to modulate mental functions though the most of data were obtained from subjective methods of assessment. Direct effects of EO on brain function remained largely to be confirmed with scientific proof. This study aimed to demonstrate quantifiable and reproducible effects of commercial vetiver (Vetiveria zizanioides) EO inhalation on sleep-waking and electroencephalogram (EEG) patterns in adult male Wistar rats. The experiments were conducted during November 2013 - February 2014.
Materials and Methods:
The following electrode implantation on the skull, control, and treated animals were subjected for EEG recording while inhaling water and vetiver EO (20 and 200 µl), respectively. Fast Fourier transform was used for analysis of EEG power spectrum.
Results:
One-way ANOVA analysis confirmed that vetiver EO inhalation significantly increased total waking and reduced slow-wave sleep time. Moreover, EO inhalation decreased alpha and beta1 activity in both frontal and parietal cortices and increased gamma activity in the frontal cortex. Changes in these frequencies began almost from the start of the inhalation.
Conclusion:
These data suggest refreshing properties of vetiver EO on electrical brain activity and alertness.
KEY WORDS: Electroencephalogram, essential oil, sleep-waking, vetiver, Vetiveria zizanioides
INTRODUCTION
Essential oils (EOs) have been used for the mental improvement since ancient times. However, most of the initial researches have focused mainly on antimicrobial activity [1,2]. Therefore, various physiological effects of EOs have also been consistently confirmed [3,4]. There are many contemporary applications of EOs which are still controversial especially for mood enhancements. To validate these applications, scientific proof with objective assessments is needed. Most of the direct EO mechanisms in the central nervous system (CNS) have been obtained from animal studies. These included the effects of lavender oil inhalation on rat brain areas [5]. In addition, the effects of long-term lemon oil exposure on behavioral, hormonal, and neuronal parameters were demonstrated in rats [6]. In particular, the induction of dopamine release by green odor from rat striatal brain slices may suggest the underlying mechanisms of the EO that regulate reward, mood and attention [7]. Treatment with EO from Arachis hypogaea L. stem and leaf extract led to the increase in adenosine triphosphate levels in the brain of sleep-deprived rats [8]. This might explain central mechanism of sedative effects of the EO.
Recently, the application of EOs for therapeutic purposes has been progressively increasing. Significant improvement in sleep quality was confirmed in postpartum women [9]. Similar results in postpartum women were also seen for lavender fragrance EO [10]. Moreover, increased quality of sleep and reduced anxiety level were observed in patients with coronary artery disease who received lavender EO inhalation [11]. In nursing care for residents who suffered from dementia, anxiety and disturbed sleep patterns, beneficial effects of lavender EO were reported [12]. However, these findings were not well-accepted because aromatherapy has not been well evidence-based practice. Hence, further research with more scientific methodology is, in particular, needed in terms of efficacy confirmation of EO application.
Vetiveria zizanioides, an aromatic perennial grass, is widely grown in many tropical countries. Vetiver EO can be extracted using hydrodistillation of its roots. Vetiver EO exhibited an in vitro scavenging activity with β-vetivenene, β-vetivone, and α-vetivone as strong antioxidative constituents [13]. Until recently, vetiver EO was found to produce antioxidant activity against some oxidative stress effects [14].
However, effects of vetiver EO remained to be elucidated in terms of psychological and behavioral activities. The present study aimed to examine the effects of vetiver EO inhalation on sleep-waking and EEG patterns in rats. To mimic the realistic condition, extremely low amounts of vetiver EO were blown with controlled flow rate of ambient air for the inhalation. Following electrode implantation on the skull of rats, electroencephalography was used to record electrical brain wave of animals while receiving vetiver EO inhalation. For analysis, fast Fourier transform (FFT) was used to calculate EEG power to reflect the oscillatory rhythmicity of the brain as a function of frequency. In general, brain wave synchronization was expressed in power of either broad frequency spectrum or discrete frequency bands.
MATERIALS AND METHODS
Animal Surgery
Male Wistar rats weighing 300-350 g (approximately 3-month-old) provided by the Southern Laboratory Animal Facility (Prince of Songkla University, Songkhla, Thailand) were housed in standard environmental conditions (23-25°C, 50-55% humidity and 12/12 hrs light/dark cycle) with freely access to food pellets and water ad libitum. The experiments were conducted during November 2013 - February 2014. All the tests were performed during 9.00 AM - 3.00 PM. The experimental protocols in the present study were approved by the Animals Ethical Committee of PSU (MOE 0521.11/1079).
A surgery process was performed as previously described [15]. Briefly, animals were anesthetized with an intramuscular injection of 60 mg/kg Zoletil® 100 (Virbac, Thailand Co. Ltd.). Stainless steel screw electrodes were stereotaxically implanted in the frontal cortex (AP; +3, ML; 3) and the parietal cortex (AP; –4, ML; 4) on the left side skull. The reference and ground electrodes were placed at midline over the cerebellum. Bipolar electromyogram (EMG) electrodes were inserted into bilateral dorsal neck muscles. All electrodes were secured in place with acrylic resin (Unifast Trad, Japan).
Vetiver EO Analyses
The vetiver EO was purchased from the Doi Kham shop, the Royal Project Foundation in 2012. It was diluted with dichloromethane and analyzed by a DSQII gas chromatograph equipped with a quadrupole mass analyzer (ThermoScientific, USA). Thermo Xcalibur 2.2 software was used for data acquisition. The analysis was performed using a ZB-5MS, 5% diphenyl-95% polydimethylsiloxane capillary column (30 m × 0.25 mm I. D., 0.25 µm film thickness). The column temperature was initially held at 40°C and then increased to 230°C at 4°C min−1 with a final hold time of 5 min. Helium was used as a carrier gas at a constant flow rate of 1 mL min−1. About 1 µl of the diluted sample was injected in the split mode at a ratio of 1:10. Injector, MS transfer line, and ion source temperatures were set at 230°C, 240°C and 220°C, respectively. Ionization mode was electron impact (70 eV) in the m/z range 40-300.
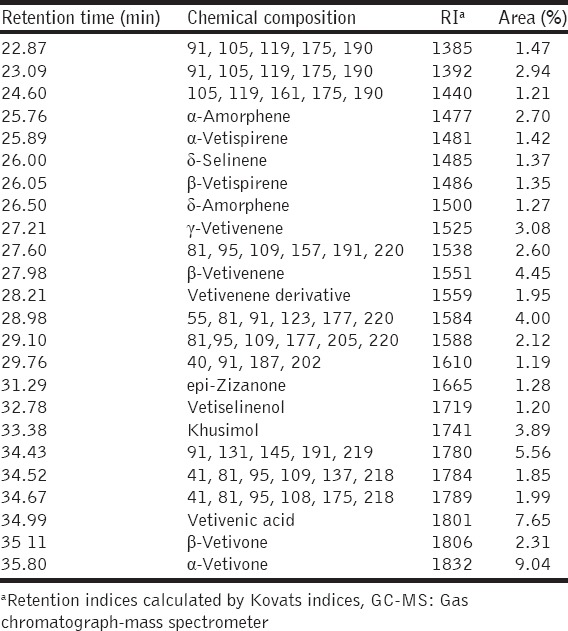
Retention indices (RI) of all constituents were calculated by the Kováts indices with references to C8-C25 n-alkanes. The oil components were identified by comparison of their RI and mass spectra with data published by Adams [16] and also by matching their mass spectra with reference spectra from The national institute of standards and technology (NIST) database. Gas chromatograph-mass spectrometer (GC-MS) chromatograms and the identified dominant volatile components, as well as their relative peak area percent values, are shown in Figure 1 and Table 1, respectively.
Figure 1.

Gas chromatograph-mass spectrometer chromatogram of vetiver essential oil
Table 1.
Chemical compositions of vetiver essential oil by GC-MS analysis
Vetiver EO Inhalation
Inhalation chamber was modified from airtight cylindrical plastic chamber with 25 and 50 cm in diameter and height, respectively. This chamber contains 2 opposite openings for connections with inlet and outlet air tubes (2.5 cm in diameter) at 5 cm above the bottom. The inlet tube was connected with a blowing apparatus for delivering ambient air or smell into the chamber and the tube on the other side was for outflow ventilation to prevent carbon dioxide buildup. The blowing apparatus was made from electric fan with the flow rate at 0.41 m3/min.
Animals were divided into control (n = 6) and 2 treated groups to receive water and vetiver EO (20 and 200 µl, n = 6 each), respectively. In general, olfactory perception is essential for detection of the olfactory stimulus but not for discrimination of olfactory intensity. Dose management of vetiver EO inhalation remained to be explored. To examine sleep response to vetiver EO inhalation, a broad range of vetiver EO quantity was chosen to ensure that it is sufficient to have CNS effects.
Animals were individually placed into the inhalation chamber. A baseline recording was performed with ambient air inhalation for 30 min and followed with either water or vetiver EO inhalation for 1 h. The EO was pipetted onto a cotton wool inside the blowing apparatus and remained for the whole period of recording. Chambers were cleaned up with 70% ethanol solution between trials to prevent transmission of olfactory cues.
EEG Recording and Data Processing
EEG signals were amplified and digitized at 400 Hz (sampling rate) by a PowerLab/4SP system (AD Instruments, Australia) with 12-bit A/D, and stored in a PC through the LabChart program software version 7.3.7 (AD Instruments, Australia). EEG signals were processed through 1.25-45 Hz digital bandpass filter. The signals were converted to power spectra by the FFT algorithm which embedded in LabChart software (Hanning window cosine transform, FFT size = 1024-point, 50% overlap). Then, the power spectra of 2.56 s sweeps of selected periods were averaged to give the power spectra of the period. The power spectra were divided into 7 frequency bands according to previous report [15,17]: Delta, 0.8-4.3 Hz; Theta, 4.7-8.2 Hz; Alpha, 8.6-12.1 Hz; Beta1, 12.5-18.0 Hz; Beta2, 18.4-30.1 Hz; and Gamma, 30.5-45 Hz. EEG powers in each frequency band of each group were averaged and expressed as percent baseline power.
Statistical Analysis
Percent baseline power of EEG signal was calculated from pre- and post-treatment periods. Baseline values were calculated from pre-treatment EEG and set to 100%, whereas posttreatment values were referenced with that of baseline to obtain percent baseline values. Data of frontal and parietal EEG were expressed as mean ± standard error of mean. Significant differences between treatments were considered using one-way ANOVA, followed by Student-Newman-Keuls method (P < 0.05, 0.01, and 0.001 for *, **, and ***, respectively). All statistics were calculated using Microsoft Excel version 2013 (Microsoft Corporation).
RESULTS
Chemical Analysis of Vetiver EO
Compositions in vetiver oil identified by GC-MS were listed in Table 1. β-vetivenene, khusimol, vetivenic acid, α-vetivone were found to be the major components of vetiver oils [Figure 1].
Effects of Vetiver EO Inhalation on Sleep-Waking State
Frontal and parietal EEG and EMG were collectively analyzed for classification of brain states [Figure 2]. Waking periods were characterized by overall desynchronized brain wave as seen in low power. In contrast, high amplitudes of low-frequency power were criteria of slow-wave sleep. Rapid eye movement (REM) sleeps were identified with the dominant parietal theta wave. EMG activity was obviously high during waking period but almost absent during REM sleep. Therefore, fragments of each brain state were summed and shown in 3 categories of brain state [Figure 3]. One-way ANOVA confirmed that EO inhalation significantly increased total awake period (F [2, 17] = 11.48, P < 0.001). The inhalation was found to result in 2 folds of waking time for both doses of vetiver EO. In contrast, the inhalation significantly decreased total slow wave sleep (F [2, 17] = 11.125, P = 0.001]. However, no significant change in REM sleep was detected.
Figure 2.

Representative electroencephalogram (EEG) and electromyogram signals from awake, slow-wave sleep and rapid eye movement sleep periods. EEG powers of signals from frontal and parietal cortices were analyzed to identify brain states
Figure 3.

Effects of vetiver essential oils inhalation on sleep-waking. **, *** = p < 0.01, p < 0.001, respectively
Effects of Vetiver EO Inhalation on EEG Power Spectrum
Power spectrum of EEG signals following the inhalation was shown in frequency domain from 1 to 45 Hz. In frontal EEG, vetiver EO inhalation was found to significantly reduce power of slow frequency range and enhance that of high-frequency range [Figure 4a]. When each frequency band was individually analyzed, significant increases were found in alpha (F [2, 19] = 3.67, P = 0.047) and beta1 (F [2, 19] = 4.33, P = 0.030) bands [Figure 4b]. It was contrast for high frequency band as gamma power was significantly increased by the inhalation (F [2, 19] = 6.149, P = 0.010).
Figure 4.

Effects of vetiver essential oils inhalation on frontal EEG power spectrum and 6 discrete band powers in frontal cortex (a and b respectively) and parietal cortex (c and d respectively). *, ** = p < 0.05, p < 0.01, respectively
Similar findings were found in parietal EEG [Figure 4c]. Vetiver EO inhalation significantly reduced powers of alpha (F [2, 19] = 6.57, P = 0.008) and beta1 (F [2, 19] = 5.902, P = 0.011) waves [Figure 4d].
Time-course Effects of Vetiver EO Inhalation
In frontal EEG, the effect of vetiver EO inhalation on slow wave activity (0.8 - 18 Hz) was particularly monitored. The results showed that the inhalation of 200 µl appeared to decrease slow wave power within a few minutes from the start [Figure 5]. The decrease of slow wave activity persisted until 1 h. On the other hand, the increase in gamma activity induced by vetiver EO inhalation was immediately observed and also remained for the whole period of recording [Figure 6].
Figure 5.

Effects of vetiver essential oils inhalation on frontal slow wave activity
Figure 6.

Effects of vetiver essential oils inhalation on frontal gamma activity
DISCUSSION
Most of previous EO studies have confirmed the effects of EOs on the CNS by measuring behavioral, hormonal, cellular, and molecular parameters. These investigations used relatively indirect techniques that detected some physiological changes following EO administration. However, they revealed the potential of EOs in modifying CNS functions that regulate reward [18], mood [19-21], attention [22], and memory and learning [23]. In particular, there are only few studies that confirmed EO effects on the CNS by using electrical brain oscillations. Recently, the findings from our previous work clearly indicated the modification of electrical brain activity induced by orange (Citrus sp.) EO inhalation [24]. Increasing of slow wave oscillation (4-12 Hz) by orange EO suggested possible anxiolytic or sedative effects reported previously [24]. These data appeared to indicate that brain oscillations reflect spontaneous and direct activity of neural network circuits associated with behavioral status. Therefore, recording of EEG or electrocorticography might be the highly sensitive method to monitor effects of EOs on neural network activity.
In folklore medicine, V. zizanioides Linn or vetiver. has been used in the treatment of various illnesses including mental and emotional symptoms. In modern scientific studies, it was claimed to reduce oxidative stress according to its antioxidative [14,25] and scavenging [26] activities. The present study demonstrated the stimulating effects of vetiver EO inhalation with increased total awake period, gamma EEG power and decreased slow wave EEG power. Alterations of electrical brain wave by EOs have been reported in animal models. Previously, inhalation of citrus EO produced EEG patterns in both frontal and parietal cortices which might be associated with anxiolytic-like effect [24]. In addition, dose-dependent increase in the power of fast wave in the hippocampus and the cerebral cortex was also produced following intraperitoneal injection of bergamot EO [27]. Altogether, these findings collectively suggest that EOs are capable of modifying CNS functions. However, EOS are supposed to be applied through inhalation as the most common route for volatile substances. Therefore, it was an ideal purpose to examine the effects of inhaled vetiver EO on EEG pattern. As shown in the results, significant changes in EEG activity were seen following vetiver EO inhalation though the weight of the EO (in cotton wool) remained almost unchanged after the test. It means that the administration system ventilated extremely low amounts of the EO through recording chamber. This suggests that inhalation is probably the most effective route of EO administration. Previously, the inhalation has been consistently found to deliver EO to various organs including the brain and produce anxiolytic-like effect [28].
There are some advantages of EEG studies. With the use of FFT, it is possible to measure power in discrete frequency ranges of the EEG spectrum. Basically, slow frequency power increases during sleep, whereas fast frequency power is associated with wakefulness [29]. Mainly, overall brain wave activity can be overviewed in broad EEG power spectrum. Power alteration of some discrete frequency bands clearly reflects specific patterns of brain activity. In general, the decrease power in slow-wave activity and/or increase power in fast wave was positively correlated with increased alertness [30,31]. Moreover, EEG technique provides continuous data that reflect time course effect of EO treatment. In the present findings, refreshing action of vetiver EO was detected immediately after the inhalation started and persisted at least 1 h. This data would be beneficial for the odor management.
Most of previous reports of vetiver studies have focused on its anxiolytic or sedative effects proposed to be through GABAergic activities [32,33]. Moreover, it was also demonstrated to produce significant anticonvulsant activity in animal model [34]. In general, anticonvulsant drugs such as benzodiazepines and valproic acid are used against seizures both in patients and in animals by enhancing GABAergic synaptic transmission [35]. These findings might suggest sedative and hypnotic properties. However, the results from the present study indicated stimulating effect of vetiver EO. Apart from its anxiolytic or sedative property, the plant has also been tested for other CNS effects. In a study using mice model of amnesia induced by scopolamine, different extracts of vetiver significantly inhibited acetylcholinesterase activity and reversed the amnesia [36]. Consistently, the ethanolic extracts of this plant were found to enhance learning and memory possibly through cholinergic transmission in the brain [32]. Altogether, vetiver appeared to be capable of inducing learning process in the brain and exploratory behavior that would probably results in increasing alertness and fast frequency oscillation.
In terms of application, alertness is one of the basic factors that would enhance physical and mental performances. Previously, treatment with EO from Acori graminei rhizome resulted in improvement of cognitive performances and increased levels of neurotransmitters especially monoamines that would enhance wakefulness [23]. The use of vetiver EO for refreshing might be an alternative choice in addition to the consumption of tea or coffee. In terminal task, human subjects who inhaled the volatile compounds of vetiver EO showed faster reaction times and stimulation of sympathetic nerve activity [37]. Its volatile compounds may help subjects to maintain performance in a visual discrimination task. Ultimately, the stimulating effects of vetiver EO might be beneficial for learning and memory processes. In conclusion, the present findings provide information that vetiver EO may be used as a stimulant to improve alertness and task performance.
ACKNOWLEDGMENTS
This work was financially supported by grants from, the Natural Product Research Center of Excellence and Department of Physiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla 90112, Thailand.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Joshi RK, Badakar V. Chemical composition and in vitro antimicrobial activity of the essential oil of the flowers of Tridax procumbens. Nat Prod Commun. 2012;7:941–2. [PubMed] [Google Scholar]
- 2.Zarai Z, Ben Chobba I, Ben Mansour R, Békir A, Gharsallah N, Kadri A. Essential oil of the leaves of Ricinus communis L. In vitro cytotoxicity and antimicrobial properties. Lipids Health Dis. 2012;11:102. doi: 10.1186/1476-511X-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goes TC, Antunes FD, Alves PB, Teixeira-Silva F. Effect of sweet orange aroma on experimental anxiety in humans. J Altern Complement Med. 2012;18:798–804. doi: 10.1089/acm.2011.0551. [DOI] [PubMed] [Google Scholar]
- 4.Chang KM, Shen CW. Aromatherapy benefits autonomic nervous system regulation for elementary school faculty in taiwan. Evid Based Complement Alternat Med. 2011;2011:946537. doi: 10.1155/2011/946537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw D, Norwood K, Leslie JC. Chlordiazepoxide and lavender oil alter unconditioned anxiety-induced c-fos expression in the rat brain. Behav Brain Res. 2011;224:1–7. doi: 10.1016/j.bbr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Ceccarelli I, Lariviere WR, Fiorenzani P, Sacerdote P, Aloisi AM. Effects of long-term exposure of lemon essential oil odor on behavioral, hormonal and neuronal parameters in male and female rats. Brain Res. 2004;1001:78–86. doi: 10.1016/j.brainres.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Kako H, Fukumoto S, Kobayashi Y, Yokogoshi H. Effects of direct exposure of green odour components on dopamine release from rat brain striatal slices and PC12 cells. Brain Res Bull. 2008;75:706–12. doi: 10.1016/j.brainresbull.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Zu X, Zhang Z, Xiong G, Liao T, Qiao Y, Li Y, et al. Sedative effects of Arachis hypogaea L. stem and leaf extracts on sleep-deprived rats. Exp Ther Med. 2013;6:601–5. doi: 10.3892/etm.2013.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirghafourvand M, Charandabi SM, Hakimi S, Khodaie L, Galeshi M. Effect of orange peel essential oil on postpartum sleep quality: A randomized controlled clinical trial. Eur J Integr Med. 2015 doi: 10.1016/j.eujim.2015.07.044. (In Press) [Google Scholar]
- 10.Keshavarz Afshar M, Behboodi Moghadam Z, Taghizadeh Z, Bekhradi R, Montazeri A, Mokhtari P. Lavender fragrance essential oil and the quality of sleep in postpartum women. Iran Red Crescent Med J. 2015;17:e25880. doi: 10.5812/ircmj.17(4)2015.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karadag E, Samancioglu S, Ozden D, Bakir E. Effects of aromatherapy on sleep quality and anxiety of patients. Nurs Crit Care. 2015 doi: 10.1111/nicc.12198. [DOI] [PubMed] [Google Scholar]
- 12.Johannessen B. Nurses experience of aromatherapy use with dementia patients experiencing disturbed sleep patterns. An action research project. Complement Ther Clin Pract. 2013;19:209–13. doi: 10.1016/j.ctcp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Chen F, Wang X, Chung HY, Jin Z. Evaluation of antioxidant activity of vetiver (Vetiveria zizanioides L.) oil and identification of its antioxidant constituents. J Agric Food Chem. 2005;53:7691–5. doi: 10.1021/jf050833e. [DOI] [PubMed] [Google Scholar]
- 14.Luqman S, Kumar R, Kaushik S, Srivastava S, Darokar MP, Khanuja SP. Antioxidant potential of the root of Vetiveria zizanioides (L.). Nash. Indian J Biochem Biophys. 2009;46:122–5. [PubMed] [Google Scholar]
- 15.Cheaha D, Keawpradub N, Sawangjaroen K, Phukpattaranont P, Kumarnsit E. Effects of an alkaloid-rich extract from Mitragyna speciosa leaves and fluoxetine on sleep profiles, EEG spectral frequency and ethanol withdrawal symptoms in rats. Phytomedicine. 2015;22:1000–8. doi: 10.1016/j.phymed.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Adam RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4th ed. Carol Stream, IL, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 17.Cheaha D, Sawangjaroen K, Kumarnsit E. Characterization of fluoxetine effects on ethanol withdrawal-induced cortical hyperexcitability by EEG spectral power in rats. Neuropharmacology. 2014;77:49–56. doi: 10.1016/j.neuropharm.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Khatibi A, Haghparast A, Shams J, Dianati E, Komaki A, Kamalinejad M. Effects of the fruit essential oil of Cuminum cyminum L. on the acquisition and expression of morphine-induced conditioned place preference in mice. Neurosci Lett. 2008;448:94–8. doi: 10.1016/j.neulet.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Tildesley NT, Kennedy DO, Perry EK, Ballard CG, Wesnes KA, Scholey AB. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav. 2005;83:699–709. doi: 10.1016/j.physbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Moss M, Cook J, Wesnes K, Duckett P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int J Neurosci. 2003;113:15–38. doi: 10.1080/00207450390161903. [DOI] [PubMed] [Google Scholar]
- 21.Lehrner J, Marwinski G, Lehr S, Johren P, Deecke L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol Behav. 2005;86:92–5. doi: 10.1016/j.physbeh.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Ilmberger J, Heuberger E, Mahrhofer C, Dessovic H, Kowarik D, Buchbauer G. The influence of essential oils on human attention. I: Alertness. Chem Senses. 2001;26:239–45. doi: 10.1093/chemse/26.3.239. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Han T, Yu CH, Rahman K, Qin LP, Peng C. Ameliorating effects of essential oil from Acori graminei Rhizoma on learning and memory in aged rats and mice. J Pharm Pharmacol. 2007;59:301–9. doi: 10.1211/jpp.59.2.0016. [DOI] [PubMed] [Google Scholar]
- 24.Kwangai J, Hiranyachattada S, Wattapiromsakul C, Kumarnsit E. Modification of electrical brain wave by Citrus sp. Essential oil inhalation. J Physiol Biomed Sci. 2013;26:5–8. [Google Scholar]
- 25.Saikia D, Parveen S, Gupta VK, Luqman S. Anti-tuberculosis activity of Indian grass KHUS (Vetiveria zizanioides L. Nash) Complement Ther Med. 2012;20:434–6. doi: 10.1016/j.ctim.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Luqman S, Kumar R, Srivastava S, Darokar MP, Lal RK, Bahl JR, et al. Hydroxyl radical scavenging activity of aromatic grass khus (Vetiveria zizanioides) J Med Aromat Plant Sci. 2008;30:320–4. [Google Scholar]
- 27.Rombolà L, Corasaniti MT, Rotiroti D, Tassorelli C, Sakurada S, Bagetta G, et al. Effects of systemic administration of the essential oil of bergamot (BEO) on gross behaviour and EEG power spectra recorded from the rat hippocampus and cerebral cortex. Funct Neurol. 2009;24:107–12. [PubMed] [Google Scholar]
- 28.Satou T, Murakami S, Matsuura M, Hayashi S, Koike K. Anxiolytic effect and tissue distribution of inhaled Alpinia zerumbet essential oil in mice. Nat Prod Commun. 2010;5:143–6. [PubMed] [Google Scholar]
- 29.Bagetta G, De Sarro G, Priolo E, Nisticò G. Ventral tegmental area: Site through which dopamine D2-receptor agonists evoke behavioural and electrocortical sleep in rats. Br J Pharmacol. 1988;95:860–6. doi: 10.1111/j.1476-5381.1988.tb11715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrier J, Paquet J, Fernandez-Bolanos M, Girouard L, Roy J, Selmaoui B, et al. Effects of caffeine on daytime recovery sleep: A double challenge to the sleep-wake cycle in aging. Sleep Med. 2009;10:1016–24. doi: 10.1016/j.sleep.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Riba J, Anderer P, Morte A, Urbano G, Jané F, Saletu B, et al. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol. 2002;53:613–28. doi: 10.1046/j.1365-2125.2002.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nirwane AM, Gupta PV, Shet JH, Patil SB. Anxiolytic and nootropic activity of Vetiveria zizanioides roots in mice. J Ayurveda Integr Med. 2015;6:158–64. doi: 10.4103/0975-9476.146548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajasekhar CH, Kokila BN, Rajesh B. Potential effect of Vetiveria Zizanioides root extract and essential oil on phenobarbital induced sedation-hypnosis in Swiss albino mice. Int J Exp Pharmacol. 2014;4:89–93. [Google Scholar]
- 34.Gupta R, Sharma KK, Afzal M, Damanhouri ZA, Ali B, Kaur R, et al. Anticonvulsant activity of ethanol extracts of Vetiveria zizanioides roots in experimental mice. Pharm Biol. 2013;51:1521–4. doi: 10.3109/13880209.2013.799710. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald RL, McLean MJ. Anticonvulsant drugs: Mechanisms of action. Adv Neurol. 1986;44:713–36. [PubMed] [Google Scholar]
- 36.Velmurugan C, Shajahan SK, Ashok Kumar BS, Vijaya Kumar S, Anitha Priyadharshini R, Sujith T. Memory and learning enhancing activity of different extracts of roots of Vetiveria zizanioides. Int J Novel Trends Pharm Sci. 2014;4:174–82. [Google Scholar]
- 37.Matsubara E, Shimizu K, Fukagawa M, Ishizi Y, Kakoi C, Hatayama T, et al. Volatiles emitted from the roots of Vetiveria zizanioides suppress the decline in attention during a visual display terminal task. Biomed Res. 2012;33:299–308. doi: 10.2220/biomedres.33.299. [DOI] [PubMed] [Google Scholar]


